- EN - English
- CN - 中文
Unlocking Bio-Instructive Polymers: A Novel Multi-Well Screening Platform Based on Secretome Sampling
解锁生物诱导聚合物:基于分泌组采样的新型多孔筛选平台
(*contributed equally to this work) 发布: 2024年02月20日第14卷第4期 DOI: 10.21769/BioProtoc.4939 浏览次数: 2668
评审: Geoffrey C. Y. LauSrajan Kapoor

相关实验方案

基于人外周血单个核细胞(PBMCs)和浆细胞样树突细胞(pDCs)的宿主靶向抗病毒药物(HTA)筛选方案
Zhao Xuan Low [...] Pouya Hassandarvish
2025年03月05日 3068 阅读

用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2351 阅读
Abstract
Biomaterials are designed to interact with biological systems to replace, support, enhance, or monitor their function. However, there are challenges associated with traditional biomaterials’ development due to the lack of underlying theory governing cell response to materials’ chemistry. This leads to the time-consuming process of testing different materials plus the adverse reactions in the body such as cytotoxicity and foreign body response. High-throughput screening (HTS) offers a solution to these challenges by enabling rapid and simultaneous testing of a large number of materials to determine their bio-interactions and biocompatibility. Secreted proteins regulate many physiological functions and determine the success of implanted biomaterials through directing cell behaviour. However, the majority of biomaterials’ HTS platforms are suitable for microscopic analyses of cell behaviour and not for investigating non-adherent cells or measuring cell secretions. Here, we describe a multi-well platform adaptable to robotic printing of polymers and suitable for secretome profiling of both adherent and non-adherent cells. We detail the platform's development steps, encompassing the preparation of individual cell culture chambers, polymer printing, and the culture environment, as well as examples to demonstrate surface chemical characterisation and biological assessments of secreted mediators. Such platforms will no doubt facilitate the discovery of novel biomaterials and broaden their scope by adapting wider arrays of cell types and incorporating assessments of both secretome and cell-bound interactions.
Key features
• Detailed protocols for preparation of substrate for contact printing of acrylate-based polymers including O2 plasma etching, functionalisation process, and Poly(2-hydroxyethyl methacrylate) (pHEMA) dip coating.
• Preparations of 7 mm × 7 mm polymers employing pin printing system.
• Provision of confined area for each polymer using ProPlate® multi-well chambers.
• Compatibility of this platform was validated using adherent cells [primary human monocyte–derived macrophages (MDMs)) and non-adherent cells (primary human monocyte–derived dendritic cells (moDCs)].
• Examples of the adaptability of the platform for secretome analysis including five different cytokines using enzyme-linked immunosorbent assay (ELISA, DuoSet®).
Graphical overview
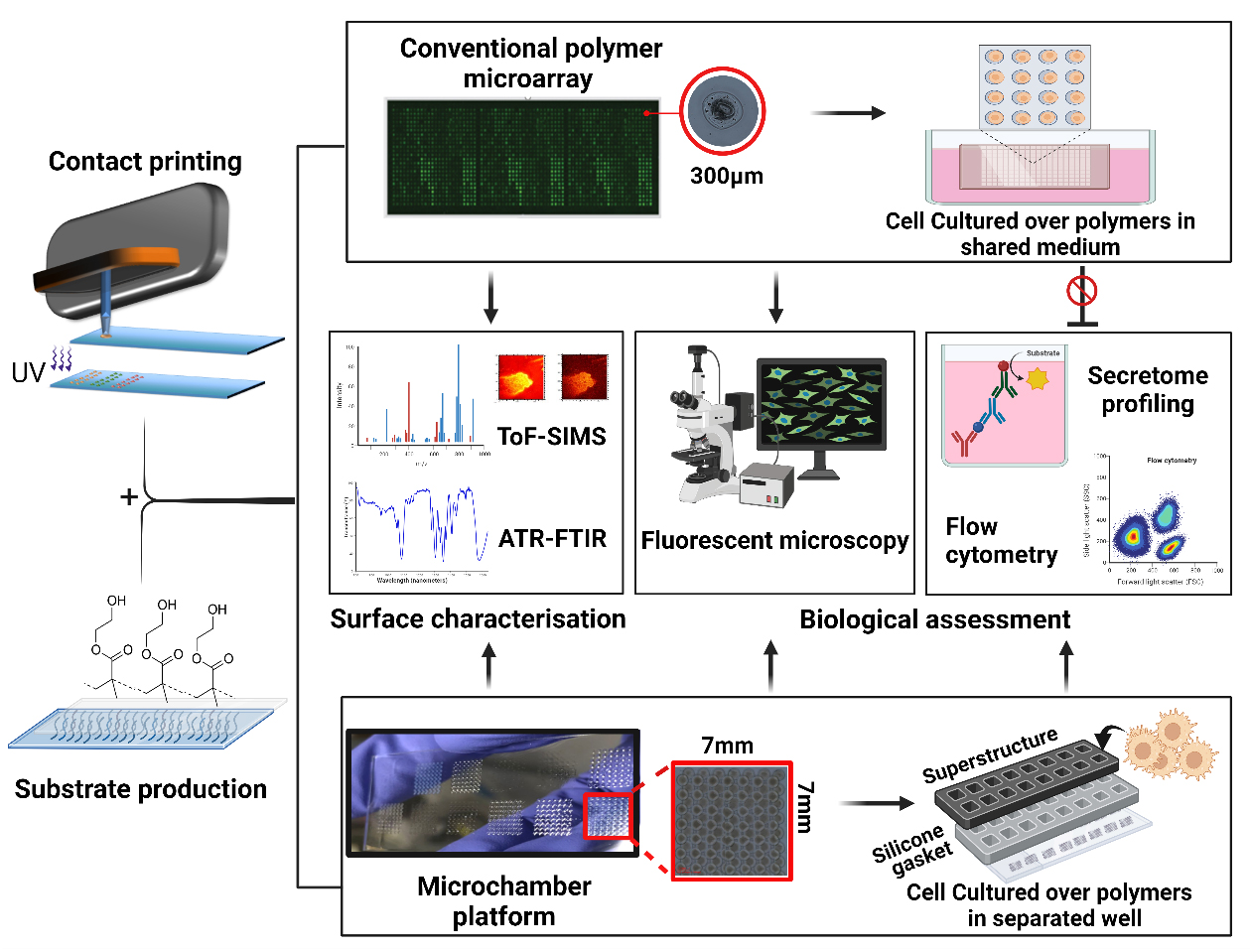
Background
Biomaterials play a pivotal role in the development of advanced medical devices, drug delivery systems, and regenerative therapies to enhance patient outcomes and quality of life [1–4]. However, current drawbacks include device-associated infection, adverse immune responses, and in-service degradation that can collectively reduce implant performance [5–7]. Rational design of bio-instructive materials remains unattainable due to our limited understanding of material–biological interactions [1]. As a result, screening is a commonly employed approach for discovering and optimising novel biomaterials with a desired biological function, such as pro- or anti-inflammatory properties. High-throughput screening (HTS) strategies have accelerated the development of biomaterials by enabling researchers to rapidly analyse a large number of samples or conditions in a systematic manner [8]. A commonly used HTS approach for biomaterials is the use of printed polymer microarrays, which have been employed to screen cell responses to materials, allowing reproducible control over cellular behaviour [9]. Key examples are screening for scalable synthetic cultureware for human pluripotent stem cells [10], polymers to modulate the foreign body response and wound healing [11,12], and a new class of bacteria-attachment-resistant materials [13]. In these studies, thousands of materials are robotically printed and in situ ultraviolet (UV)-polymerised on a single slide. This approach is used to discern specific biological interactions by culturing cells directly on the polymer array surfaces within a shared culture medium [14,15]. Despite the success of such polymer microarray platforms in biomaterials’ discovery, the secreted biochemical signals are an untapped resource in understanding cellular phenotypes. Paracrine signalling is also a worry requiring follow-up studies on individually scaled up polymers before potential hits can be verified. Understanding cell-surface interactions requires probing biochemical cues, in addition to biomechanical, topographical, and material chemistry/bio-interfacial cues. Hence, without knowledge of the secretome profile of the cells following their interaction with materials, the understanding of the functionality of the polymer is incomplete. Furthermore, polymer microarrays are limited to the assessment of cells with strong adhesion abilities. Notably, for cells such as dendritic and T cells, which possess weaker substrate adhesion tendencies, attributing their phenotypical changes to a particular polymer spot would be incredibly challenging [16–18].
In response to these limitations of the polymer microarray, we developed a novel platform that combines existing arraying technology such as contact and inkjet printing, multi-well microchambers, and high-throughput secretome profiling. The platform benefits from reusable superstructures (ProPlate®) that are mountable on the printed glass substrates. This offers the provision of confined culture volumes, each designated for a distinct polymer condition with a surface area and volume compatible with cell culture for a few days. This separation allows us to closely study the mediators released by cells, helping us understand how these materials interact with cells and the mechanisms of the biomolecules involved. In addition, this system allows for high-throughput surface characterisation such as time-of-flight secondary ion mass spectrometry, x-ray photoelectron spectroscopy, and attenuated total reflectance–Fourier transform infrared spectroscopy (ATR-FTIR), ensuring the accurate identification and validation of controlling moieties [19]. Together, these analytical approaches have the potential to enhance the discovery of new biomaterials and improve our understanding of biomaterials–cells interface. Yet, there are constraints to the throughput and time-related aspects of this platform. In a single operational cycle, the platform demonstrates the capacity to fabricate ten slides, each accommodating 16 unique chemical compositions, resulting in the synthesis of 160 distinct polymer entities, thereby yielding 8,960 discrete polymer spots. By way of comparison, a polymer microarray system can generate 17,280 polymer deposition sites from 576 chemistries in triplicates across ten slides [20]. Nevertheless, this limited output can be ameliorated through the implementation of 64-well superstructures from GraceBio-Labs and the application of multiplexing methodologies as alternatives to conventional techniques such as enzyme-linked immunosorbent assay (ELISA). Here, we provide a step-by-step description of methodologies and troubleshooting aspects involved in developing this platform, including preparation of substrate, fabrication of multi-well chambers, and provision of confined area for each polymer condition, followed by relevant examples of the anticipated outcomes such as chemical characterisation and biological assessments of the secreted soluble mediators.
Materials and reagents
Reagents
Oxygen (O2) gas (any vendor)
Molecular sieves (4 Å) (VWR International, catalog number: 215-283-8)
Toluene (Fisher Scientific, catalog number: T290-4)
3-glycidoxypropyltrimethoxysilane (GPTMS) (Sigma-Aldrich, catalog number: 440167-500mL)
Argon gas (any vendor)
Acetone (Fisher Scientific, catalog number: A949SK-4)
Poly(2-hydroxyethyl methacrylate) (pHEMA) (Sigma-Aldrich, catalog number: P3932-25g)
Deionised distilled water (Milli-Q®) (Millipore, Sigma-Aldrich, USA)
Ethanol (Sigma-Aldrich, catalog number: 1009862500)
Ethoxyethyl acrylate (EOEA) (Sigma-Aldrich, USA, catalog number: 106-74-1)
Dimethylformamide (DMF) (Fisher Scientific, catalog number: AA22915K7)
Photoinitiator (2,2-dimethoxy-2-phenylacetophenone) (DMPA) (Sigma-Aldrich, catalog number: 19611-8)
Isopropanol (Sigma-Aldrich, catalog number: 563935)
Tween® 20 (Sigma-Aldrich, catalog number: 9005-64-5)
Phosphate buffer solution (PBS) (Sigma-Aldrich, catalog number: D8537)
Trypan blue dye (any vendor)
ToxiLightTM assay (Lonza, USA, catalog number: LT17-217)
ELISA DuoSet® (R&D Systems, USA) (TNF-α, catalog number: DY210; IL-10, catalog number: DY217; TGF-β1, catalog number: DY240; CCL-18, catalog number: DY394; IL-6, catalog number: DY206; IL-12, catalog number: DY1270)
Laboratory supplies
Glass slides (25 mm × 75 mm) (VWR, catalog number: 631-1553)
Glass beaker (any vendor)
Needles (21 G, 120 mm) (Fisher Scientific, catalog number: 10438881)
Needles (21 G, 40 mm) (Sterican® Safety Needle, catalog number: Z118044)
Plastic syringe 50 mL (any vendor)
Crystallizing dish (Pyrex, capacity 1,200 mL)
Parafilm® (Sigma-Aldrich, catalog number: P7543)
Falcon tube 50 mL (Sigma-Aldrich, catalog number: T2318)
Polypropylene 384-well plate (Corning, product number: 3656)
Glass Pasteur pipette (VWR, catalog number: 612-1702p)
Weighing boats (any vendor)
Glove box (MBRAUN, Germany)
Microarray print head 16 pins (BioDot, USA)
Plastic snap clips (GraceBio-Labs, catalog number: 204830)
4-well rectangular plate for slides (Thermo Fisher, NuncTM, catalog number: 267060)
Equipment
Glass funnel (any vendor)
Support stand (A-frame) (any vendor)
Laboratory clamp (any vendor)
Hotplate (any vendor)
Stainless steel rack for glass slides (Sigma-Aldrich, catalog number: Z710989)
Fume hood (any vendor)
Plasma etcher (Diener, model: Nano LFG40)
Vacuum oven (Thermo Scientific, model: Vacutherm)
Sonicator (any vendor)
Dip-coater (Holmarc, model: HO-TH-01 dip-coater)
Water contact angle measurement apparatus (KSV Instruments, model: CAM 100)
Polypropylene pipette tips (any vendor)
Pipette (any vendor)
Electric pipettor controller (any vendor)
Weighing scale (0.01 g and 0.0001 g sensitivity) (any vendor)
Spatula (any vendor)
Pin printing workstation (BioDot, model: XYZ3200)
Microarray ceramic pin 500 µm (LabNEXT Inc, model: XtendTM)
UV lamp (365 nm, any vendor)
O2 sensor (Cambridge Sensotec, model: rapidox 1100)
Optical profiler (KLA, model: ZetaTM-300)
Multi-well chambers (16-well ProPlate®) (GraceBio-Labs, catalog number: 244864)
Software and datasets
BioDot AxSysTM (BioDot, USA)
MicroLab expert (Agilent, USA)
Spectrus Processor (ACD lab, USA)
GraphPad Prism software (Version 10.0.2, USA)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Fateh, S., Alromaihi, R. A., Ghaemmaghami, A. M. and Alexander, M. R. (2024). Unlocking Bio-Instructive Polymers: A Novel Multi-Well Screening Platform Based on Secretome Sampling. Bio-protocol 14(4): e4939. DOI: 10.21769/BioProtoc.4939.
分类
生物工程 > 生物医学工程
免疫学 > 免疫细胞功能 > 细胞因子
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









