- EN - English
- CN - 中文
Generation of Human Induced Pluripotent Stem Cell (hiPSC)-Derived Astrocytes for Amyotrophic Lateral Sclerosis and Other Neurodegenerative Disease Studies
产生人类诱导多能干细胞 (hiPSC) 衍生的星形胶质细胞,用于研究肌萎缩侧索硬化症和其他神经退行性疾病
发布: 2024年02月20日第14卷第4期 DOI: 10.21769/BioProtoc.4936 浏览次数: 6612
评审: Marina Sánchez PetidierAnthony FlamierAnonymous reviewer(s)
Abstract
Astrocytes are increasingly recognized for their important role in neurodegenerative diseases like amyotrophic lateral sclerosis (ALS). In ALS, astrocytes shift from their primary function of providing neuronal homeostatic support towards a reactive and toxic role, which overall contributes to neuronal toxicity and cell death. Currently, our knowledge on these processes is incomplete, and time-efficient and reproducible model systems in a human context are therefore required to understand and therapeutically modulate the toxic astrocytic response for future treatment options. Here, we present an efficient and straightforward protocol to generate human induced pluripotent stem cell (hiPSC)-derived astrocytes implementing a differentiation scheme based on small molecules. Through an initial 25 days, hiPSCs are differentiated into astrocytes, which are matured for 4+ weeks. The hiPSC-derived astrocytes can be cryopreserved at every passage during differentiation and maturation. This provides convenient pauses in the protocol as well as cell banking opportunities, thereby limiting the need to continuously start from hiPSCs. The protocol has already proven valuable in ALS research but can be adapted to any desired research field where astrocytes are of interest.
Key features
• This protocol requires preexisting experience in hiPSC culturing for a successful outcome.
• The protocol relies on a small molecule differentiation scheme and an easy-to-follow methodology, which can be paused at several time points.
• The protocol generates >50 × 106 astrocytes per differentiation, which can be cryopreserved at every passage, ensuring a large-scale experimental output.
Graphical overview
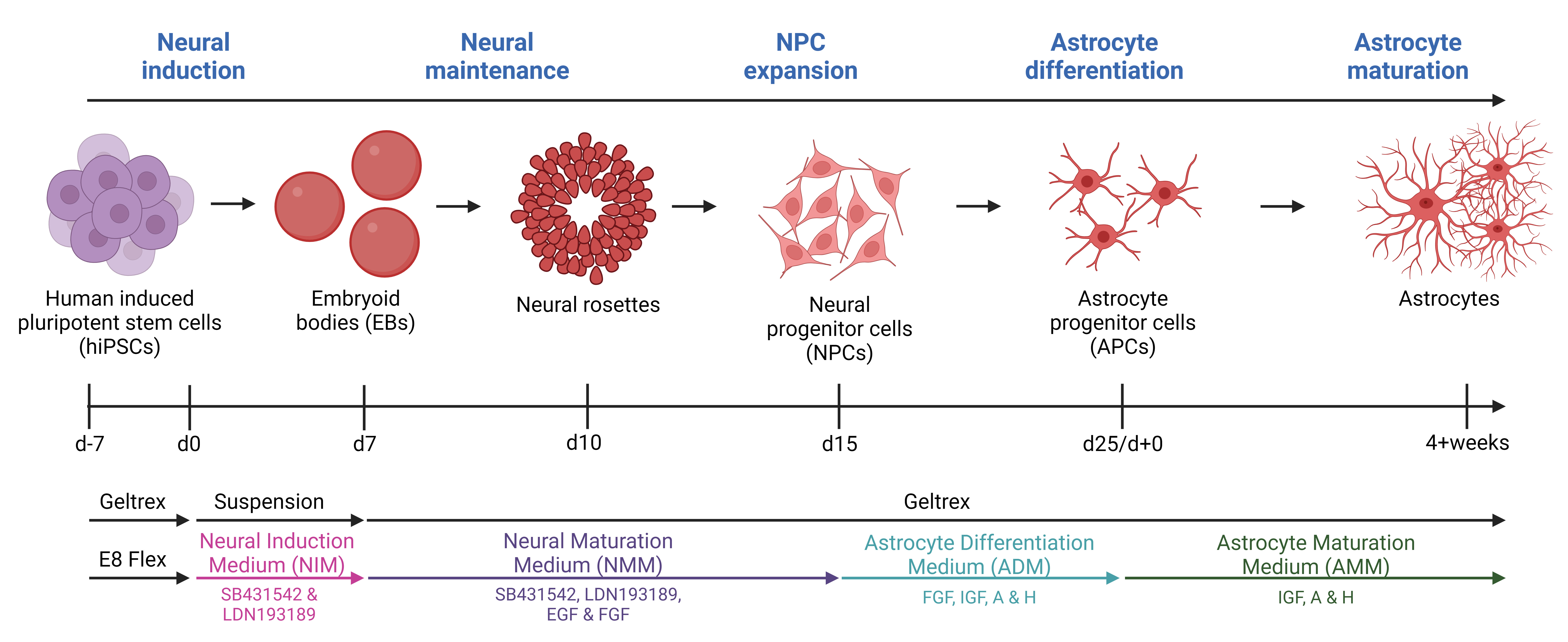
Background
Neurodegenerative diseases affect millions of people worldwide and as the average population age increases, there is a corresponding rise in the number of patients. Amyotrophic lateral sclerosis (ALS) is one of these neurodegenerative diseases. ALS, the most prevalent motor neuron disorder among adults, affects approximately 2 out of 100,000 individuals across a wide age range, encompassing cases from teenagers to the elderly [1]. Ten percent of cases are caused by inherited familial mutations, while 90% have no family history and are therefore classified as sporadic [2]. Hallmarks of ALS include toxic protein aggregations, axonal transport impairments, DNA damage, and glial reactivity, leading to extensive motor neuron death [3–7]. This causes muscle atrophy, paralysis, and death of patients typically within 2–5 years after symptom onset, and currently, there is no cure [1]. As with many other neurodegenerative diseases, the focus has been on unraveling the underlying disease mechanisms behind the apparent (motor)neuronal cell death; however, the widespread glial reactivity has recently resulted in a shift from the neurocentric perspective towards increased appreciation of the role of glial cells. Astrocytes are shown to be key players in neurodegeneration [8]. As one of the most abundant glial cell types in the central nervous system, astrocytes govern the support and homeostatic maintenance of neurons and their surroundings [9]. Under physiological conditions, astrocytes have many functions including neurotransmitter modulation, nutritional distribution, ion, pH, and water homeostasis, blood–brain barrier regulation, and trophic support [9,10]. However, in ALS and other neurodegenerative diseases, astrocytes lose these supportive characteristics and take on a more toxic reactive role [10]. Considerable knowledge about the pathophysiology of ALS involving astrocytes has been gained through the use of animal models. However, it is important to acknowledge that, like all models, they come with their inherent limitations [10]. Animal models often rely on overexpression of human mutant genes, which despite showing various disease-relevant mechanisms, often fail to translate to a human context [11]. Importantly, overexpression models also exclude the large and important group of sporadic patients. Furthermore, human astrocytes exhibit larger sizes, more intricate branching structures, and a greater extent of synapse interactions compared to their rodent counterparts [12–14]. As a result, the field of animal research necessitates reinforcement from human in vitro models, and human-induced pluripotent stem cells (hiPSCs) present as highly promising candidates. With their ability for self-renewal and indefinite proliferation, as well as their possibility to generate any cell type, they hold significant potential. Several protocols for generating hiPSC-derived astrocytes exist, but many of the protocols are complex and require long timelines to reach the state of full astrocyte differentiation [15–21]. Our protocol is based on a 25-day-long differentiation followed by a 4+ week maturation. The differentiation is based on a dual inhibition of SMAD signaling pathway with the introduction of 3D culturing [22] to generate neural progenitor cells (NPCs) and a modified astrocyte differentiation protocol from Shaltouki et al. [23] to generate astrocytes [24,25]. After four weeks of maturation, > 95% of the hiPSC-derived astrocyte population is positive for typical astrocyte markers (S100β, AQP4, SOX9, and ALDH1L1) [24]. Importantly, the hiPSC-derived astrocytes retain their morphology, marker expression, and functionality, when cocultured with hiPSC-derived motor neurons [24].
Materials and reagents
Biological materials
Human induced pluripotent stem cells (hiPSCs) (generated in-house [5])
Reagents
Essential 8TM Flex medium kit (E8 Flex medium) (Thermo Fisher Scientific, Gibco, catalog number: A28583-01)
GeltrexTM Matrix (Geltrex) (Thermo Fisher Scientific, Gibco, catalog number: A1413301)
DMEM/F-12 +Lglut, +HEPES (DMEM/F-12) (Thermo Fisher Scientific, Gibco, catalog number: 11330032)
Penicillin-Streptomycin (Pen/Strep) (5,000 U/mL) (Thermo Fisher Scientific, Gibco, catalog number: 15070063)
RevitaCellTM supplement (100×) (Thermo Fisher Scientific, Gibco, catalog number: A2644501)
DPBS, no calcium, no magnesium (Thermo Fisher Scientific, Gibco, catalog number: 14190144)
Collagenase type IV powder (Thermo Fisher Scientific, Gibco, catalog number: 17104019)
SB431542 (Tocris Bioscience, catalog number: 1614; product format: 10 mM in ethanol)
LDN193189 (Stemgent, catalog number: 04-0074-02; product format: 10 mM in solution)
NeurobasalTM medium (Thermo Fisher Scientific, Gibco, catalog number: 21103049)
B-27TM supplement minus vitamin A (50×) (Thermo Fisher Scientific, Gibco, catalog number: 12587-010)
N-2 Supplement (100×) (Thermo Fisher Scientific, Gibco, catalog number: 17502048)
L-Glutamine (200 mM) (Thermo Fisher Scientific, Gibco, catalog number: 25030024)
Aqua ad iniectabilia (injectable water) (B. Braun, catalog number: 2351744)
Recombinant murine FGF-basic (FGF) (Peprotech, catalog number: 450-33; product format: 10 µg/mL in DPBS)
Recombinant human epidermal growth factor (EGF) (ProSpec, catalog number: CYT-217; product format: 100 µg/mL in injectable water)
Accutase® solution (Sigma-Aldrich, catalog number: A6964)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, Gibco, catalog number: 10270106)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650)
MEM non-essential amino acids solution (NEAA) (100×) (Thermo Fisher Scientific, Gibco, catalog number: 11140050)
L-Ascorbic acid (Sigma-Aldrich, catalog number: A4403; product format: 200 µM in injectable water)
Recombinant human IGF-1 (IGF) (Peprotech, catalog number: 100-11; product format: 100 µg/mL in injectable water)
Human Activin A recombinant protein (A) (Thermo Fisher Scientific, Gibco, catalog number: PHC9564; product format: 10 µg/mL in DPBS)
Recombinant human Heregulin β-1 (H) (Peprotech, catalog number: 100-03, product format: 250 µg/mL in injectable water)
Sodium pyruvate (100 mM) (Thermo Fisher Scientific, Gibco, catalog number: 11360070)
Trypan blue solution, 0.4% (Thermo Fisher Scientific, Gibco, catalog number: 15250061)
Ethanol absolute ≥ 99.8% (VWR, catalog number: 20821.296)
Isopropanol, 99.5% (Thermo Fisher Scientific, catalog number: 184130010)
Solutions
E8 Flex medium (see Recipes)
Geltrex coating (see Recipes)
Collagenase type IV (10× and 1× solutions) (see Recipes)
Neural induction medium (NIM) (see Recipes)
Neural maturation medium (NMM) (see Recipes)
Astrocyte differentiation medium (ADM) (see Recipes)
Astrocyte maturation medium (AMM) (see Recipes)
Recipes
E8 Flex medium
Thaw the frozen Essential 8 TM Flex supplement from the Essential 8TM Flex medium kit at room temperature for approximately 1 h or at 2–8 °C overnight. Protect the supplement from light, as it is light sensitive. Mix the thawed supplement by gently inverting the vial a couple of times and then aseptically transfer the entire contents of the Essential 8TM Flex supplement to the bottle of Essential 8 TM Flex basal medium. Swirl the bottle to mix. E8 Flex medium can be stored at 2–8 °C for up to two weeks.
Reagent Final concentration Volume Essential 8TM Flex basal medium 98% 500 mL Essential 8TM Flex supplement (50×) 2% 10 mL Total 100% 510 mL Geltrex coating
It is important to keep all components at ≤ 2–8 °C during the preparation of the Geltrex coating to prevent premature solidification. To ensure this, first prepare 24.75 mL of 2–8 °C DMEM/F-12 in a 50 mL conical tube and then collect the Geltrex aliquot from -20 °C. Transfer approximately 0.5 mL of 2–8 °C DMEM/F-12 from the prepared 50 mL conical tube to the Geltrex aliquot, pipette up and down to dissolve the frozen aliquot, and transfer approximately 0.75 mL of the solution back to the 50 mL conical tube. Repeat the process a few times to transfer the entire content of the Geltrex aliquot to the 50 mL conical tube. Mix well. Geltrex coating can be stored at 2–8 °C for one week.
Reagent Final concentration Volume DMEM/F-12 99% 24.75 mL Geltrex 1% 250 µL Total 100% 25 mL Collagenase type IV (10× and 1× solutions)
First, prepare a stock concentration (10×): Dilute 1 g of collagenase type IV powder in 100 mL of DMEM/F-12 and filter sterilize. Aliquot the 10× solution in 10 mL/aliquot. Next, prepare the working concentration (1×): Dilute 10 mL of the stock concentration (10×) with 90 mL of DMEM/F-12 to make a 1× solution and filter sterilize. Aliquot the 1× solution in 12.5 mL/aliquot. Both 10× and 1× aliquots can be stored at -20 °C for ≤ 6 months. Bring collagenase type IV (1×) to room-temperature before use.
Reagent Final concentration Quantity or Volume DMEM/F-12 100% 100 mL Collagenase type IV (powder) 10 mg/mL (10×) 1 g DMEM/F-12 90% 90 mL Collagenase type IV (10×) 1 mg/mL (1×) 10 mL Neural induction medium (NIM): d0-d6
Prepare ~500 mL of bulk solution of E8 Flex medium and Pen/Strep and filter sterilize. E8 Flex + Pen/strep can be stored at 2–8 °C for two weeks. To prepare NIM, make an aliquot of the required volume for the day of E8 Flex + Pen/Strep solution and add SB431542 and LDN193189 fresh on the day of use. Filter sterilize and bring the NIM solution to room temperature before use.
Reagent Final concentration Volume E8 Flex medium 100% 500 mL Pen/Strep 1% 5 mL SB431542 10 µM see note LDN193189 0.1 µM see note Neural maturation medium (NMM): d7-d15
Prepare > 200 mL of bulk solution of basic medium (DMEM/F12, neurobasal medium, Pen/Strep, B-27 minus vitamin A, N-2, and L-Glutamine) and filter sterilize. Basic medium can be stored at 2–8 °C for four weeks. To prepare NMM, make an aliquot of the required volume for the day of basic medium solution and add SB431542, LDN193189, FGF, and EGF fresh on the day of use. Filter sterilize and bring the NMM solution to 37 °C before use.
Reagent Final concentration Volume (for 200 mL) DMEM/F-12 47.5% 95 mL Neurobasal medium 47.5% 95 mL Pen/Strep 1% 2 mL B-27 minus vitamin A 2% 4 mL N-2 1% 2 mL L-Glutamine 1% 2 mL SB431542 10 µM see note LDN193189 0.1 µM see note FGF 10 ng/mL see note EGF 10 ng/mL see note Astrocyte differentiation medium (ADM): d16-d25
Prepare >200 mL of bulk solution of basic medium (neurobasal medium, Pen/Strep, N-2, NEAA, and L-Ascorbic acid) and filter sterilize. Basic medium can be stored at 2–8 °C for four weeks. To prepare ADM, make an aliquot of the required volume for the day of basic medium solution and add FGF, IGF, A, and H fresh on the day of use. Filter sterilize and bring the ADM solution to 37 °C before use.
Reagent Final concentration Volume (for 200 mL) Neurobasal medium 97% 194 mL Pen/Strep 1% 2 mL N-2 1% 2 mL NEAA 1% 2 mL L-Ascorbic acid (200 µM) 0.8 µM 800 µL FGF 10 ng/mL see note IGF 200 ng/mL see note A 10 ng/mL see note H 10 ng/mL see note Astrocyte maturation medium (AMM): d25+
Prepare 500 mL of bulk solution of basic medium (DMEM/F12, neurobasal medium, Pen/Strep, N-2, NEAA, L-Ascorbic acid, L-Glutamine, sodium pyruvate, and FBS) and filter sterilize. Basic medium can be stored at 2–8 °C for four weeks. To prepare AMM, make an aliquot of the required volume for the day of basic medium solution and add IGF, A, and H fresh on the day of use. Filter sterilize and bring the AMM solution to 37 °C before use.
Reagent Final concentration Volume (for 500 mL) DMEM/F-12 46.3% 231.5 mL Neurobasal medium 46.3% 231.5 mL Pen/Strep 1% 5 mL N-2 1% 5 mL NEAA 1% 5 mL L-Ascorbic acid (200 µM) 0.8 µM 2 mL L-Glutamine 1% 5 mL Sodium pyruvate 1% 5 mL FBS 2% 10 mL IGF 200 ng/mL see note A 10 ng/mL see note H 10 ng/mL see note Laboratory supplies
Cell culture flasks, 25 cm2, cell-repellent surface (T25 non-adherent flask) (Greiner Bio-One, catalog number: 690980)
Cell culture multi-well plates (6-well plate) (Greiner Bio-One, catalog number: 657160)
25 mL sterile reservoirs (Thermo Fisher Scientific, catalog number: 95128095) or 50 mL sterile reservoirs (InvitroLab, catalog number: IV-6002)
Cell scrapers (TH Geyer, catalog number: 7696760)
15 mL conical tubes (TH Geyer, catalog number: 7696714)
50 mL conical tubes (Greiner Bio-One, catalog number: 227261)
150 mL vacuum filtration devices, pore 0.22 µm (Jet Biofil, catalog number: FCF010004)
500 mL vacuum filtration devices, pore 0.22 µm (Jet Biofil, catalog number: FPE204500)
2 mL cryovials (Maxxline, catalog number: MLC2B)
5 mL serological pipettes (Greiner Bio-One, catalog number: 606180)
10 mL serological pipettes (Greiner Bio-One, catalog number: 607180)
25 mL serological pipettes (Greiner Bio-One, catalog number: 760160-TRI)
Sterile PES syringe filters (Thermo Fisher Scientific, catalog number: 15206869)
50 mL 3-part syringes (Chirana T. Injecta, catalog number: CH03050LL)
10 µL pipette tips (TH Geyer, catalog number: 7695881)
20 µL pipette tips (TH Geyer, catalog number: 7695882)
200 µL pipette tips (TH Geyer, catalog number: 7695884)
1,250 µL pipette tips (TH Geyer, catalog number: 7695887)
CountessTM cell counting chamber slides (Thermo Fisher Scientific, Invitrogen, catalog number: C10283)
Equipment
NordicSafe® Class II biological safety cabinet (ESCO, catalog number: NC2-L)
CellXpert® C170i CO2 incubator (Eppendorf, catalog number: 6734)
EVOSTM XL Core inverted microscope (objectives: 4×, 10×, 20×) (Thermo Fisher Scientific, catalog number: AMEX1000)
Laboratory centrifuge with rotors for 15 and 50 mL conical tubes (Biosan, catalog number: LMC-3000)
Water bath (37 °C) (Julabo, catalog number: TW8)
FinnpipetteTM F2 GLP pipetting kit 2 (Thermo Fisher Scientific, catalog number: 11835850)
Pipetboy acu 2 (Integra, catalog number: 1550179)
Mr. FrostyTM freezing container (Thermo Fisher Scientific, catalog number: 5100-0001)
CountessTM II FL automated cell counter (Thermo Fisher Scientific, catalog number: AMQAF1000)
Racks
Liquid nitrogen (N2) tank
Freezer (-20 °C)
Refrigerator (2–8 °C)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Stoklund Dittlau, K., Chandrasekaran, A., Freude, K. and Van Den Bosch, L. (2024). Generation of Human Induced Pluripotent Stem Cell (hiPSC)-Derived Astrocytes for Amyotrophic Lateral Sclerosis and Other Neurodegenerative Disease Studies. Bio-protocol 14(4): e4936. DOI: 10.21769/BioProtoc.4936.
分类
神经科学 > 神经系统疾病 > 神经退行性病变
干细胞 > 多能干细胞 > 细胞分化
细胞生物学 > 细胞分离和培养 > 细胞分化
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












