- EN - English
- CN - 中文
Addressing the Role of Conventional CD8αβ+ T Cells and CD4+ T Cells in Intestinal Immunopathology Using a Bone Marrow–Engrafted Model
使用骨髓移植模型探讨传统 CD8αβ+ T 细胞和 CD4+ T 细胞在肠道免疫病理学中的作用
发布: 2024年02月20日第14卷第4期 DOI: 10.21769/BioProtoc.4934 浏览次数: 2968
评审: Andrea GramaticaMarco Di GioiaAnonymous reviewer(s)
Abstract
Inflammatory bowel disease (IBD) is characterized by an aberrant immune response against microbiota. It is well established that T cells play a critical role in mediating the pathology. Assessing the contribution of each subset of T cells in mediating the pathology is crucial in order to design better therapeutic strategies. This protocol presents a method to identify the specific effector T-cell population responsible for intestinal immunopathologies in bone marrow–engrafted mouse models. Here, we used anti-CD4 and anti-CD8β depleting antibodies in bone marrow–engrafted mouse models to identify the effector T-cell population responsible for intestinal damage in a genetic mouse model of chronic intestinal inflammation.
Key features
• This protocol allows addressing the role of CD4+ or CD8αβ+ in an engrafted model of inflammatory bowel disease (IBD).
• This protocol can easily be adapted to address the role of other immune cells or molecules that may play a role in IBD.
Keywords: T cell (T细胞)Background
Inflammatory bowel disease (IBD) is a chronic and recurring inflammation in the gastrointestinal tract that affects more than 1.3 million people in Europe (Zhao et al., 2021). IBD includes ulcerative colitis and Crohn’s disease. It is common for patients with ulcerative colitis and Crohn's disease to experience diarrhea, rectal bleeding, abdominal pain, fatigue, and weight loss. Furthermore, bearers of this debilitating disease are at high risk of inflammation-associated cancers such as colorectal cancer (Kim and Chang, 2014). Although it is widely accepted that IBD is characterized by an excessive immune response to microbiota, the etiology of IBD remains a mystery, being crucial to create novel models that can unravel the intricate microbial, molecular, and cellular interactions contributing to the onset of IBD.
Several immune-regulatory mechanisms prevent IBD. Particularly, SMAD4 in T cells, a significant downstream component of the cytokine TGF-β pathway, prevents mice from developing chronic intestinal inflammation. Indeed, researchers have demonstrated that ablation of SMAD4 specifically in T cells using a CD4-CRE system leads to severe chronic intestinal inflammation (Hahn et al., 2011; Kim et al., 2006). This inflammation typically emerges around 5–7 months of age. T cells deficient in SMAD4 contribute to intestinal inflammation. However, the model employed to illustrate this is based on the CD4-CRE system. As both CD4+ and CD8αβ+ T cells undergo a stage expressing both CD4 and CD8 markers during their generation in the thymus, the deletion of SMAD4 occurs in both CD4+ and CD8αβ+ T cells. It is crucial to specify which subset of T cells is responsible for mediating the observed pathology in the context of the immunopathology observed in IBD.
At present, no animal model entirely recapitulates Crohn’s disease or ulcerative colitis. Models such as the SMAD4 model present the benefit of not using chemical agents such as dextran sulfate sodium (DSS) and closely mimicking the chronicity of the inflammation without external intervention, in contrast to chemical-induced colitis models. Although useful, colitis models based on the transfer of naïve T cells into mice lacking adaptive immunity (SCID or RAG mice) do not recapitulate the entire onset of IBD. Indeed, those models lack an adaptive immune system. The SMAD4 model provides some interesting insights that closely resemble what happens in humans, such as impairment on TGFβ signaling pathway (Monteleone et al., 2001).
The present protocol offers an approach to address the implication of different populations of T cells in IBD. It utilizes a bone marrow–engrafted mouse model and specific anti-CD4 and anti-CD8β depleting antibodies to eliminate conventional CD8αβ+ and CD4+ T cells, sparing unconventional T cells such as TCRγδ T cells (Figure 1). This protocol can be adapted for the study of other cellular and molecular mechanisms potentially implicated in IBD.
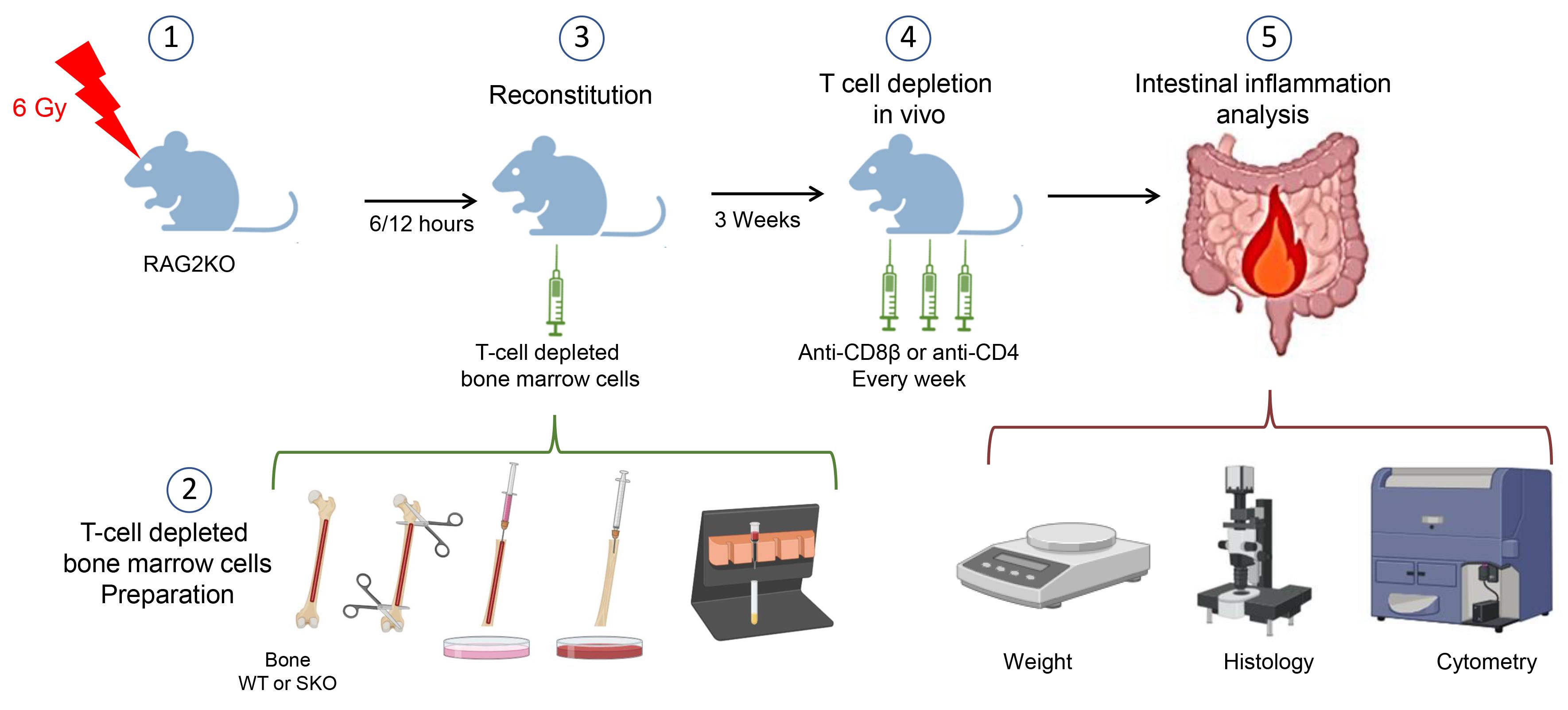
Figure 1. Schematic model of the procedures. This figure was created with BioRender.com.
Materials and reagents
Biological materials
Animals
All mice were on the C57BL/6J background and were maintained in PPAC, a specific pathogen-free animal facility of the Cancer Research Center of Lyon (CRCL), France. RAG2-KO mice were obtained from Charles River Laboratories and bred and maintained in the animal facility (specific pathogen-free). Mice were older than 8 weeks when used for the experiment. CD4-Cre+ Smad4floxed/floxed (SKO) mice and littermate CD4-Cre-Smad4floxed/floxed (WT) mice were the donors of bone marrow cells. In terms of gut inflammation development, no discernible difference was observed between males and females. However, the mice used should be of the same age and sex in the same experiment.
Materials
Pipette tips 10 μL, 20 μL, 200 μL, and 1 mL (Starlab)
5 mL sterile syringe (BD, catalog number: 3095971)
50 mL centrifuge tube (Falcon, catalog number: 352070)
15 mL conical centrifuge tube (Falcon, catalog number: 352097)
Petri dishes (100 × 20 mm) (FALCON, catalog number 353003)
10 mL serological pipettes (SARTSTEDT, catalog number: 86.1254.001)
25 G needle (BD, catalog number: 300600)
BD U-100 insulin syringe with a 29 G needle (BD, catalog number: 324891)
Neubauer chamber (KOVA international, catalog number: 87144 F)
Tubes 1.5 mL, autoclaved (STARLAB, catalog number: S1615-5500)
Nylon meshes with a mesh size of 70 μm (SEFAR NITEX, catalog number: 03-80/29), sterilized by autoclaving
Reagents
Surface disinfectants (Anios, SURFA’SAFE)
LD columns (Miltenyi Biotec, catalog number: 130-042-901)
Anti-CD8α BV605 (clone 53-6.7) (BioLegend, catalog number: 100748)
Anti-CD8b Alexa 700 (clone YTS156.7.7) (BioLegend, catalog number: 126618)
Anti-CD4 Brilliant Violet 711 (clone RM4.5) (BD, catalog number: 563726)
Anti-CD3e Brillant Violet 650 (clone 17A2) (BioLegend, catalog number: 100229)
Anti-CD45 APC-Cy7 (clone 30-F11) (BioLegend, catalog number: 103116)
Anti-CD103 e450 (clone 2E7) (eBioscience, catalog number: 48-1031-82)
Anti-Ly6g PE/Dazzle594 (clone 1A8) (BioLegend, catalog number: 127648)
Anti-CD11b APC (clone M1/70) (BioLegend, catalog number: 553312)
Anti-TCRgd FITC (clone GL3) (BD, catalog number: 553177)
LIVE/DEAD™ Fixable Yellow Dead Cell Stain kit (Life technologies, catalog number: L34968)
InVivoMAb anti-mouse CD8β (Lyt 3.2) (Clone 53-5.8) (BioXcell, catalog number: BE0223)
InVivoMAb rat IgG1 isotype control, anti-horseradish peroxidase (Clone HRPN) (BioXcell, catalog number: BE0088)
InVivoMAb anti-mouse CD4 (Clone GK1.5) (BioXcell, catalog number: BE0003-1)
InVivoMAb rat IgG2b isotype control, anti-keyhole limpet hemocyanin (Clone LTF-2) (BioXcell, catalog number: BE0090)
Fetal bovine serum (FBS) (Gibco®, catalog number: A5256701)
RPMI-1640 medium, GlutaMAX™ supplement (Life Technologies, Gibco, catalog number: 61870036)
HEPES buffer solution, 1 M (Gibco, catalog number: 15630-080)
Hank’s buffered salt solution (HBSS) (Gibco, catalog number: 14175-053)
Phosphate buffered saline (PBS 10×) (Life Technologies, Gibco, catalog number: 14200-059)
Ethanol 70% (Alcool modifié COOPER®) used as an antiseptic
Ethanol absolute (VWR Chemicals, catalog number: 20821.365)
CD3ϵ microbead kit, mouse (Miltenyi Biotec, catalog number: 130-094-973)
Trypan blue solution, 0.4% (Thermo Fisher, catalog number: 15250061)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906)
EDTA, pH 8.0 (EUROMEDEX, catalog number: EU0084)
Isoflurane
Gentamicin (50 mg/mL) (Gibco, catalog number: 15750-037)
Penicillin-Streptomycin (Life Technologies, catalog number: 15140-122)
Solutions
RPMI complete media (see Recipes)
Intra-epithelial lymphocyte enrichment buffer (see Recipes)
MACS buffer (see Recipes)
Recipes
RPMI complete media
RPMI-1640 enriched with 10% heat-inactivated FBS, 10 mM HEPES, and penicillin-streptomycin (1,000 U/mL).
MACS buffer
PBS 1× with 2 mM EDTA and 2% BSA
Equipment
Irradiator (Elekta Synergy® or any alternate irradiator)
Sterile cell culture hood (Thermo, model: HERAsafe KS 12 1.2m/41489710/2013)
QuadroMACS magnetic cell separator (Miltenyi Biotec, catalog number: 130-090-976)
Microscope (Zeiss, model: Primovert)
Serological pipettes [SARSTEDT, catalog number: 86.1253.001 (5 mL), 86.1254.001 (10 mL), and 86.1685.001 (25 mL)]
Pipette controller (INTEGRA, model: pipetboy)
Surgical scissors and forceps (Dutscher, catalog number: 005064, 005065, 711198, 005072)
Ice bucket (Dutscher, catalog number: 830116)
Anesthesia chamber (Tem Sega® or any alternate instrument)
Weigh balance (OHAUS®, model: CS200)
Pie-shape chambers (model equivalent of pie cage for X-ray irradiation from Bioseb Lab instrument®)
Animal transfer box [InnoVive, model: M-BTM (bottom) and MSX4 (lid)]
LSRII flow cytometer (BD Biosciences, model: LSR Fortessa HTS)
Centrifuge (Thermo Fisher, model: Heraeus Megafuge)
Software
FlowJoTM software (BD Biosciences) (v. 10.9, 2023)
GraphPad Prism (v. 10.1.2, 2023)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Aoudi, A., Labiad, O., Igalouzene, R., Mejri, O., Sanchez, M. and Soudja, S. (2024). Addressing the Role of Conventional CD8αβ+ T Cells and CD4+ T Cells in Intestinal Immunopathology Using a Bone Marrow–Engrafted Model. Bio-protocol 14(4): e4934. DOI: 10.21769/BioProtoc.4934.
分类
免疫学 > 免疫细胞染色 > 流式细胞术
细胞生物学 > 细胞移植 > 免疫细胞过继转移
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














