- EN - English
- CN - 中文
SUrface SEnsing of Translation (SUnSET), a Method Based on Western Blot Assessing Protein Synthesis Rates in vitro
翻译表面检测 (SUnSET),一种基于蛋白质印迹的体外蛋白质合成率评估方法
发布: 2024年02月05日第14卷第3期 DOI: 10.21769/BioProtoc.4933 浏览次数: 5068
评审: Chiara AmbrogioMayank GautamRupkatha Banerjee
Abstract
As the most energy- and metabolite-consuming process, protein synthesis is under the control of several intrinsic and extrinsic factors that determine its fine-tuning to the cellular microenvironment. Consequently, variations in protein synthesis rates occur under various physiological and pathological conditions, enabling an adaptive response by the ce•ll. For example, global protein synthesis increases upon mitogenic factors to support biomass generation and cell proliferation, while exposure to low concentrations of oxygen or nutrients require translational repression and reprogramming to avoid energy depletion and cell death. To assess fluctuations in protein synthesis rates, radioactive isotopes or radiolabeled amino acids are often used. Although highly sensitive, these techniques involve the use of potentially toxic radioactive compounds and require specific materials and processes for the use and disposal of these molecules. The development of alternative, non-radioactive methods that can be easily and safely implemented in laboratories has therefore been encouraged to avoid handling radioactivity. In this context, the SUrface SEnsing of Translation (SUnSET) method, based on the classical western blot technique, was developed by Schmidt et al. in 2009. The SUnSET is nowadays recognized as a simple alternative to radioactive methods assessing protein synthesis rates.
Key features
• As a structural analogue of aminoacyl-transfer RNA, puromycin incorporates into the elongating peptide chain.
• Detection of puromycin-labeled peptides by western blotting reflects translation rates without the need for radioactive isotopes.
• The protocol described here for in vitro applications is derived from the SUnSET method originally published by Schmidt et al. (2009).
Background
As a structural analogue of aminoacyl-tRNA, the aminonucleoside antibiotic puromycin is incorporated through non-hydrolysable peptide bounding into the growing peptide chain along the elongation process (Nathans, 1964). While high concentrations block the elongation phase and hence translation, at low doses the overall translation rate of protein synthesis remains unchanged. Consequently, the rate of formation of puromycin-labeled peptides mirrors the rate of protein translation. Taking advantage of this property, 3H-puromycin labeling was first used in 1979 to assess the rate of protein synthesis in various tissues in vivo under nutrient- and protein-poor diets (Nakano and Hara, 1979). The SUnSET (SUrface SEnsing of Translation) technique was developed 30 years later by Schmidt and colleagues to detect variations in protein synthesis rates in cultured cells by western blotting (Schmidt et al., 2009). This method was then coupled with other techniques (fluorescence-activated cell sorting or immunohistochemistry) to assess protein synthesis at different scales. Further developments, notably based on the Clik-it technology combined with O-propargyl puromycin (OP-Puro), a puromycin analogue, enable visualization of puromycilated proteins and assessment of elongation rates in tissues (Morral et al., 2020). The main advantage of using puromycin and its analogues is that it does not require radioactive isotopes such as 35S-methionine, historically used to measure protein synthesis rates, with comparable analytical performance (Schmidt et al., 2009). Because proteostasis defects are associated with a variety of chronic diseases (cancer, tissue fibrosis, inflammatory syndromes, etc.) or aging, it is necessary to monitor the changes in protein synthesis rates in response to a variety of stressors in order to better understand the translational reprogramming underlying cell adaptation. The following protocol describes a SUnSET method (Figure 1) suitable for assessing protein synthesis rates in lysates from cells grown in vitro by conventional western blot analysis.
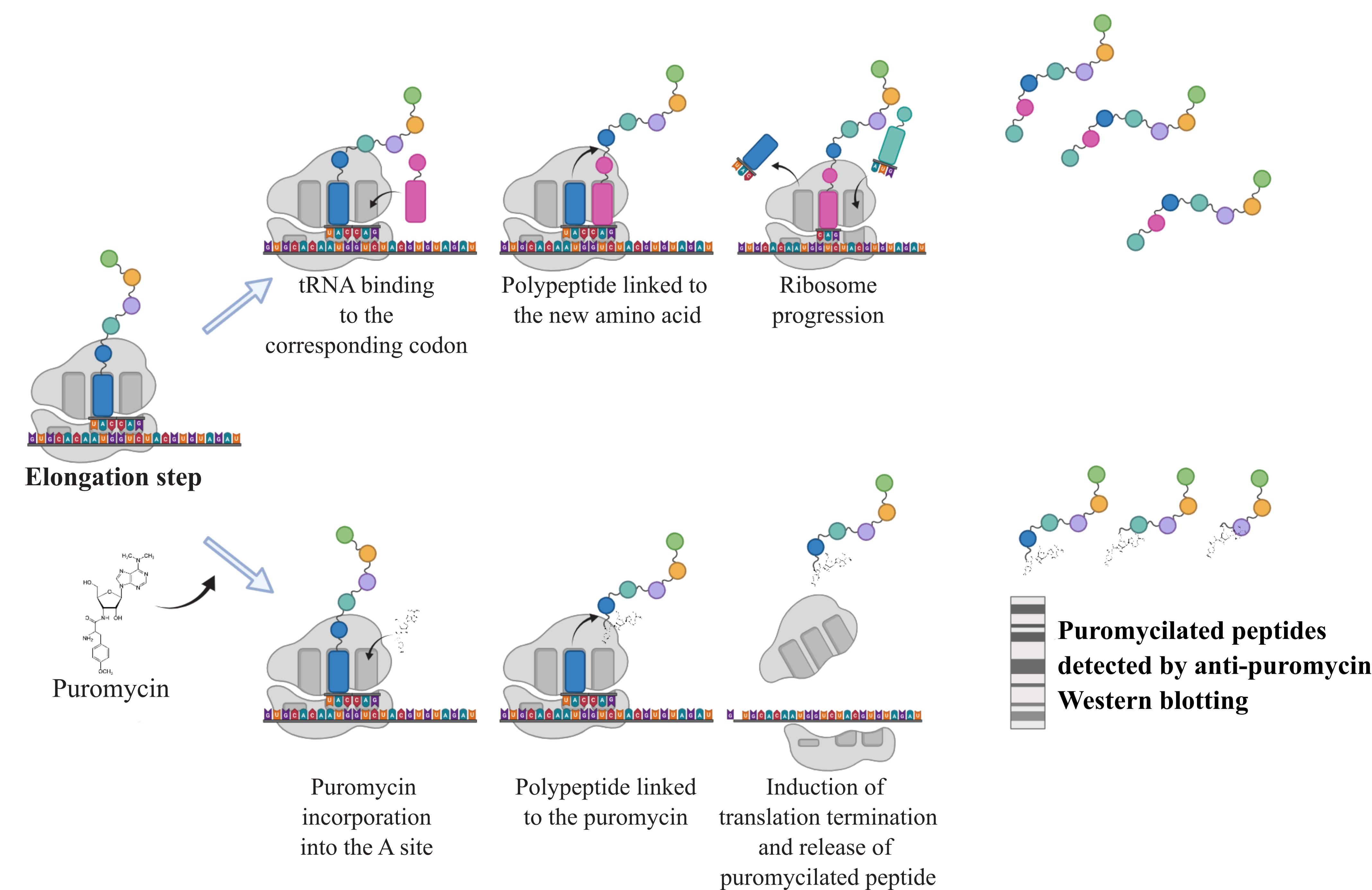
Figure 1. Principle of the SUnSET assay. During the elongation step, addition of puromycin leads to its incorporation into the A site of the ribosome. The transfer and linkage of the polypeptide to the puromycin cause the termination of translation releasing puromycilated proteins that can subsequently be detected by western blotting against the puromycin.
Materials and reagents
Biological materials
Cell lines of interest obtained from the American Type Culture Collection (ATCC). In this study, we used the colon cancer cell line HCT116.
Reagents
Puromycin (Sigma-Aldrich, catalog number: P9620)
PBS (Sigma-Aldrich, catalog number: D1408)
Complete anti-protease (Roche, catalog number: 11836145001)
Dry milk (Régilait, catalog number: 304934416704)
Bovine serum albumin (BSA) (Roche, catalog number: 10735094001)
DC Protein Assay kit (Bio-Rad, catalog number: 5000111)
Prestained protein ladder (Euromedex, catalog number: 06P-0111)
Immobilion Forte western substrate (Merck Millipore, catalog number: WBLUF0500)
Ponceau red solution (Sigma-Aldrich, catalog number: 141194)
Mouse anti-puromycin antibody (clone 12D10) (Merck Millipore, catalog number: MABE343)
Mouse anti-tubulin antibody (clone DM1A) (Sigma, catalog number: T6199)
HRP-conjugated anti-mouse secondary antibody (Cell Signaling Technology, catalog number: 7076)
Stripping buffer (Thermo Scientific, catalog number: 21059)
Solutions
RIPA protein lysis buffer 2× (see Recipes)
Tris-buffered saline-Tween (TBS-T) (see Recipes)
TBS-T 5% BSA (see Recipes)
TBS-T 5% dry milk (see Recipes)
Laemmli 6× (see Recipes)
Recipes
RIPA protein lysis buffer 2×
Reagent Final concentration Tris-HCl pH 7.2 100 mM NaCl 300 mM EDTA 10 mM Sodium deoxycholate 2% SDS 20% 0.1% Triton 100× 2× Na3VO4 4 mM β glycerophosphate 20 mM NaF 20 mM Complete anti-protease 2× Dilute to 1× in H2O.
Tris-buffered saline-Tween (TBS-T)
Reagent Final concentration Tris-HCl pH 7.5 50 mM NaCl
Tween
150 mM
0.1%
Add 5% of BSA or dry milk for obtaining TBS-T 5% BSA or 5% dry milk, respectively.
Laemmli 6×
Reagent Final concentration Tris-HCl pH 6.8 0.5 M Glycerol 1% SDS 20% 2% DTT 0.6 M Bromophenol blue 0.4%
Laboratory supplies
6-well or 10 cm diameter tissue culture plates
Sterile scrapers
Microcentrifuge tubes
Plastic wrap
Equipment
Tissue culture apparatus (tissue culture hood, CO2 incubator, etc.)
Pipettes and micropipettes
Vacuum pump
Centrifuge (4 °C)
Cold room
Heat block
Western blotting apparatus (SDS-PAGE running cassette, power supply, shaker, transfer cassette, nitrocellulose membrane, etc.)
ChemiDoc imaging system (Bio-Rad, catalog number: 12003153)
Software and datasets
Fiji (National Institutes of Health)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Piecyk, M., Fauvre, J., Duret, C., Chaveroux, C. and Ferraro-Peyret, C. (2024). SUrface SEnsing of Translation (SUnSET), a Method Based on Western Blot Assessing Protein Synthesis Rates in vitro. Bio-protocol 14(3): e4933. DOI: 10.21769/BioProtoc.4933.
分类
生物化学 > 蛋白质 > 合成
分子生物学 > 蛋白质 > 表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link