- EN - English
- CN - 中文
Streamlined Adeno-Associated Virus Production Using Suspension HEK293T Cells
使用悬浮 HEK293T 细胞简化腺相关病毒的生产流程
发布: 2024年02月05日第14卷第3期 DOI: 10.21769/BioProtoc.4931 浏览次数: 3452
评审: Neha NandwaniXiaozhe DingVictor Tse
Abstract
Recombinant adeno-associated viruses (rAAVs) are valuable viral vectors for in vivo gene transfer, also having significant ex vivo therapeutic potential. Continued efforts have focused on various gene therapy applications, capsid engineering, and scalable manufacturing processes. Adherent cells are commonly used for virus production in most basic science laboratories because of their efficiency and cost. Although suspension cells are easier to handle and scale up compared to adherent cells, their use in virus production is hampered by poor transfection efficiency. In this protocol, we developed a simple scalable AAV production protocol using serum-free-media-adapted HEK293T suspension cells and VirusGEN transfection reagent. The established protocol allows AAV production from transfection to quality analysis of purified AAV within two weeks. Typical vector yields for the described suspension system followed by iodixanol purification range from a total of 1 × 1013 to 1.5 × 1013 vg (vector genome) using 90 mL of cell suspension vs. 1 × 1013 to 2 × 1013 vg using a regular adherent cell protocol (10 × 15 cm dishes).
Key features
• Adeno-associated virus (AAV) production using serum-free-media-adapted HEK293T suspension cells.
• Efficient transfection with VirusGEN.
• High AAV yield from small-volume cell culture.
Graphical overview
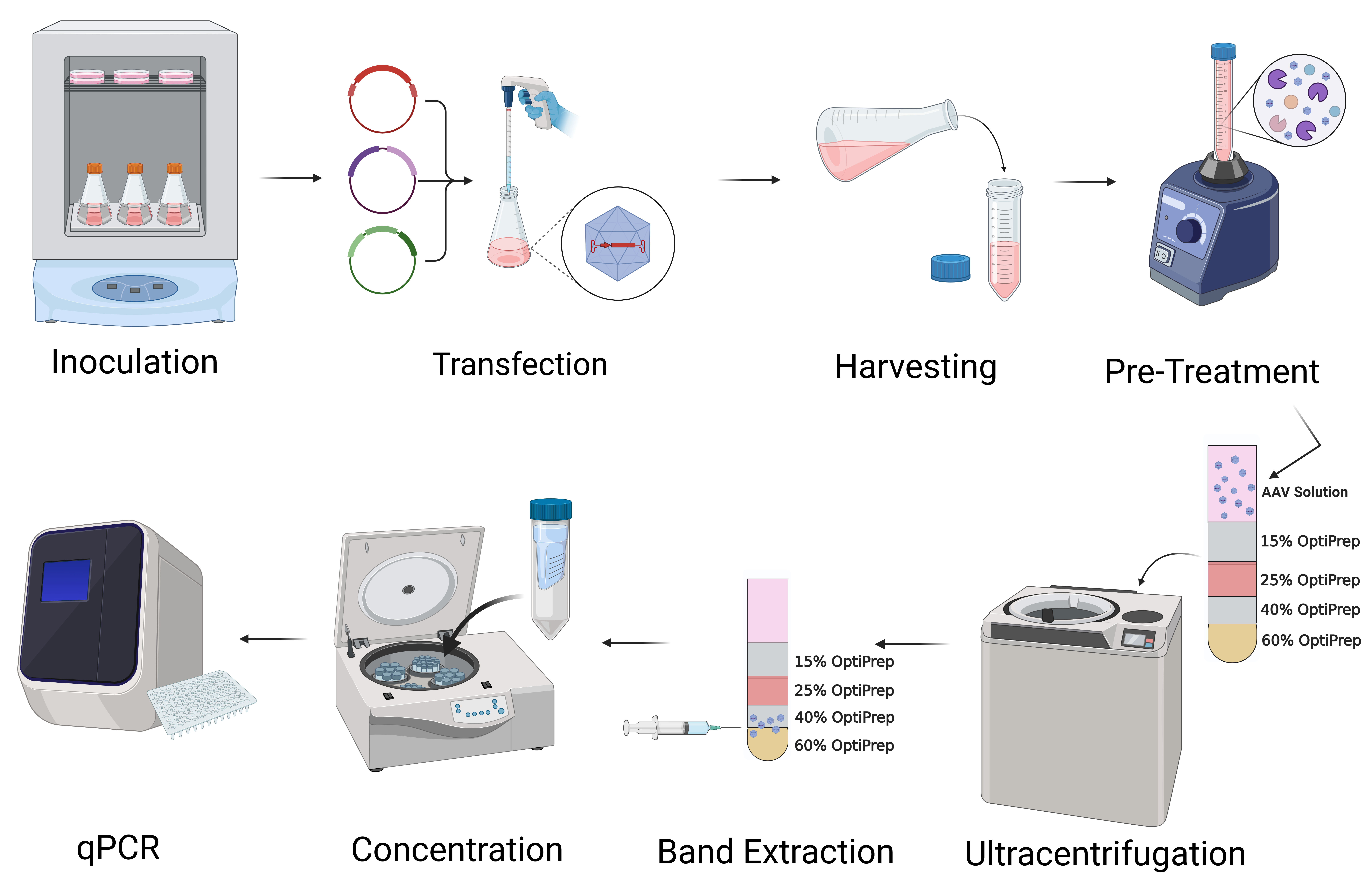
Background
The adeno-associated virus (AAV) is a non-enveloped virus belonging to the Parvoviridae family that was discovered as a contaminant in adenovirus preparations (Atchison et al., 1965). The AAV has icosahedral protein capsids of approximately 26 nm in diameter containing a single-stranded DNA genome of approximately 4.7 kb (Wang et al., 2019). Recombinant AAV (rAAV) primarily forms non-integrating episomes and sustains long-term transgene expression. It is considered to be a non-pathogenic virus and a leading viral gene transfer vector for human gene therapy (Pupo et al., 2022). Due to the discovery of numerous naturally occurring variants (> 200) and the small size of the Cap gene, capsid engineering to confer new properties to the vector has become a hot research area (Wang et al., 2019; El Andari and Grimm, 2021). Improving rAAV production that can easily be adapted to engineered serotypes is increasingly important to meet clinical demand as well as basic science needs.
Most laboratories commonly use adherent HEK293 or HEK293T cells as producer cells because transfection of adherent cells is more efficient than that of suspension cells. However, suspension cells are easier to handle and scale up, which is of particular interest to Good Manufacturing Practice (GMP) facilities. Scalable production methods include transfection of Sf9 (Mietzsch et al., 2014), HEK293 (Grieger et al., 2016), or HEK293T cells (Zhao et al., 2020). Although the insect cell-based method is more robust (Mietzsch et al., 2014), baculoviruses must be prepared before AAV production. Therefore, transfection of mammalian cells using three plasmids (a helper AdΔF6 plasmid, a Rep/Cap plasmid, and the transgene-containing AAV transfer plasmid) is more versatile and easier to adapt to newly engineered capsids. The most common transfection reagent for suspension cells is polyethyleneimine (PEI) because it is inexpensive and effective (Grieger et al., 2016; Blessing et al., 2019; Zhao et al., 2020). However, PEI is cytotoxic. Most protocols using PEI require changing media (Zhao et al., 2020; Challis et al., 2019) or diluting transfection reagent (Grieger et al., 2016; Blessing et al., 2019), while VirusGEN, a newly developed transfection reagent, is less toxic and does not require media change. We found that AAV production by transfecting HEK293T suspension cells by VirusGEN is superior to PEI and its efficiency is comparable to that of transfecting adherent cells by iMFectin poly (Deng and Oka, 2020).
There are several methods available for the purification of AAV (El Andari and Grimm, 2021). A commonly used method involves either cesium chloride (CsCl) (Ayuso et al., 2010) or iodixanol density gradient (Zolotukhin et al., 1999), which offers an advantage in separating empty and full capsids based on their density regardless of the serotype. This method allows the simultaneous purification of multiple small-scale preparations. However, scaling up or automating this process presents challenges. Another purification method is liquid chromatography. In this approach, ion exchange columns or affinity columns are connected to fast protein liquid chromatography (FPLC) or high-performance liquid chromatography (HPLC) (Nass et al., 2018; Joshi et al., 2021; Florea et al., 2023). This method is robust and preferred for clinical-grade AAV, since it can be automated and is suitable for large-scale production. However, the affinity column does not differentiate between empty and full capsids. Generally, a second column, such as an anion exchange (AEX) column, is employed to separate empty and full capsids based on the charge difference brought on by the vector genome. However, the chromatographic purification method requires adjustments and optimizations for each serotype. The choice of purification method(s) depends on various factors, including the specific AAV serotype, the downstream application, available resources, cost, and desired purity level.
Purified AAV can be characterized by several methods for quality control (QC). The most important QC assay is genome titer, which can be standardized among laboratories using reference standard material (Ayuso et al., 2014). The most popular method for this purpose is quantitative PCR (qPCR), with digital PCR (dPCR) being the most recent advancement of qPCR technology (Quan et al., 2018). Although dPCR measures absolute numbers without a standard, dPCR has a narrow dynamic range compared to qPCR due to the limited number of partitions. The conventional assay to determine empty capsids in AAV preparations is transmission electron microscope (TEM) (Grieger et al., 2006). Nonetheless, FPLC- or HPLC-AEX (Lock et al., 2012; Khatwani et al., 2021) and HPLC coupled with size exclusion column and multi-angle light scattering (McIntosh et al., 2021) have gained popularity for their simplicity and high throughput. Analytical ultracentrifugation, mass spectrometry, and charge detection mass spectrometry (Werle et al., 2021; Ebberink et al., 2022) are used to analyze empty and full capsids, including those containing partial genomes. For detecting minor DNA contaminants, such as host DNA contamination and plasmid DNA used for transfection, next-generation sequencing (Lecomte et al., 2015; Guerin et al., 2020) is employed, which can also assess vector genome integrity. The integrity of capsid proteins can be assessed by sodium-dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which detects major capsid proteins (VP1, VP2, and VP3) in a 1:1:10 ratio and other protein contaminants. However, the heterogeneity of capsid proteins has been reported. Another important QC parameter that may influence transgene expression is the infectivity of AAV. Different serotypes use different cellular docking sites, making a universal method difficult to achieve. The most accurate assay relying on AAV biological activities is the infectious center assay, while the most widely used is the median tissue infective dose (TCID50) assay, which determines genome replication by qPCR (Zen et al., 2004). Neither assay addresses cell type-specific infectivity. Although measuring intracellular vector genomes upon cell infection may not directly reflect biological activities (François et al., 2018), this method is simple and broadly applicable to any target cell. Therefore, the choice of QC methods is again dictated by the same factors described for purification methods.
In this protocol, a subclone of HEK293T cells adapted for serum-free media is transfected in suspension and AAV9 is purified by iodixanol density gradient (Zolotukhin et al., 1999). The critical determinants for transfection are cell viability and cell density. The combination of suspension cells and effective transfection reagents allows high-yield vector production [> 1 × 1013 vector genome of purified AAV9 from 90 mL of culture vs. 1 × 1013 to 2 × 1013 vg from a comparable adherence cell culture described in Deng and Oka (2020)]. In addition, hands-on time is drastically shortened compared with conventional adherent cell protocols. AAVs produced by this protocol have been characterized by qPCR, SDS-PAGE, TEM, and infectious titer assay. The quality of AAVs of different serotypes and transgenes varies and additional QCs may be required in some experiments. Using the same protocol and QCs to standardize AAVs for experiments is recommended.
Materials and reagents
Biological materials
HEK293T (ATCC, catalog number: CRL-3216) adapted for serum-free media; alternatively, request BalanCD HEK293 serum-free-media-adapted HEK293T cells (1F11S) from the corresponding author
pAdΔF6 helper plasmid (Addgene, catalog number: 112867)
pAAV2/9 Rep/Cap plasmid (Addgene, catalog number: 112865)
AV0-EF1-N-cG (control AAV transfer plasmid) (Addgene, catalog number: 192888) or AV0-EF1-N-tdT (Addgene, catalog number: 192889)
DNase I (Sigma-Aldrich, catalog number: DN25-1G)
RNase A (Thermo Fisher Scientific, catalog number: BP25391)
Forward qPCR primer such as WPRE or other vector specific primer (Sigma-Aldrich, WPRE-172 nucleotide sequence: TTTATGAGGAGTTGTGGCCC)
Reverse qPCR primer such as WPRE or other vector specific primer (Sigma-Aldrich, WPRE-392 nucleotide sequence: CAACACCACGGAATTGTCAG)
Reagents
BalanCD HEK293 media liquid or powder (Fujifilm Irvine Scientific, catalog number: 91165 or 94137, respectively)
GlutaMAX (Thermo Fisher Scientific, catalog number: 35050061)
100× Pluronic F-68 (Thermo Fisher Scientific, catalog number: 24040032)
VirusGEN AAV Transfection kit (Mirus Bio, catalog number: MIR 6750)
1 M Tris-HCl pH 8.0 (Thermo Fisher Scientific, catalog number: AAJ22638AP)
NaCl (Sigma-Aldrich, catalog number: BP35810)
MgCl2 Sigma-Aldrich, catalog number: 442611-500GM)
KCl (Sigma-Aldrich, catalog number: 1049360250)
1 M DTT (Thermo Fisher Scientific, catalog number: 11-101-3992)
Glycerol (Thermo Fisher Scientific, catalog number: J62399.AP or equivalent)
Sodium deoxycholate (Sigma-Aldrich, catalog number: D6750)
HEPES (Sigma-Aldrich, catalog number: H3784)
Sarcosyl (VWR, catalog number: D719-500G)
EDTA (Sigma-Aldrich, catalog number: E5134)
PEG8000 (Sigma-Aldrich, catalog number: P2139-2KG)
OptiPrep Density Gradient (Thermo Fisher Scientific, catalog number: NC1174452)
Phenol red (Sigma-Aldrich, catalog number: P0290-100ML)
DPBS (Gendepot, catalog number: CA008-300 or equivalent)
HyPure molecular biology grade water (Thermo Fisher Scientific, catalog number: SH3053802 or equivalent)
SYBR Green SuperMix (VWR, catalog number: 101414-168)
Sodium bicarbonate (Sigma-Aldrich, catalog number: S6014-500G)
Trypan blue (Thermo Fisher Scientific, catalog number: 15250061)
Solutions
Complete BalanCD HEK293 media (c-BalanCD HEK293) (see Recipe 1)
DNase I solution (see Recipe 2)
RNase A solution (see Recipe 3)
2 M MgCl2 (see Recipe 4)
5 M NaCl (see Recipe 5)
2.5 M NaCl (see Recipe 6)
40% PEG8000/2.5 M NaCl (see Recipe 7)
TMN (see Recipe 8)
HBS (see Recipe 9)
5% sodium deoxycholate (see Recipe 10)
PBS-MK (see Recipe 11)
PBS-NMK (see Recipe 12)
DPBS/Pluronic F-68 (see Recipe 13)
BalanCD HEK293 media—powder reconstituted (see Recipe 14)
Recipes
c-BalanCD HEK293 media
Reagent Final concentration Quantity BalanCD HEK293 [or BalanCD HEK293—powder reconstituted (see Recipe 14)] n/a 97 mL 100× GlutaMAX 4 mM 2 mL 100× Pluronic F-68 1× 1 mL Total n/a 100 mL Store this solution at 2–4 °C. See General Note 1 for alternatives to this recipe.
DNase I solution
Reagent Final concentration Quantity 1 M Tris-HCl pH 7.6 20 mM 2 mL NaCl 50 mM 0.2922 g Glycerol 50% (v/v) 50 mL 1 M DTT 1 mM 100 µL DNase I 10 mg/mL 1 g ddH2O n/a Add up to 100 mL Total n/a 100 mL Adjust pH to 7.6 (see General Note 2). See General Note 3 for more information about ddH2O. Aliquot this solution into 1.5 mL microcentrifuge tubes and store at -20 °C.
RNase A solution
Reagent Final concentration Quantity 1 M Tris-HCl pH 7.5 10 mM 1 mL NaCl 15 mM 87.66 mg RNase A 10 mg/mL 1 g ddH2O n/a Add up to 100 mL Total n/a 100 mL Adjust pH to 7.5. Aliquot this solution into 1.5 mL microcentrifuge tubes and store at -20 °C.
2 M MgCl2
Reagent Final concentration Quantity MgCl2 2 M 95.211 g ddH2O n/a Add up to 500 mL Total 2 M 500 mL Filter with a 0.22 µm pore size filter. Store this solution at room temperature.
5 M NaCl
Reagent Final concentration Quantity NaCl 5 M 292.2 g ddH2O n/a Add up to 1,000 mL Total 5 M 1,000 mL Filter with a 0.22 µm pore size filter. Store this solution at room temperature.
2.5 M NaCl
Reagent Final concentration Quantity NaCl 2.5 M 73.05 g ddH2O n/a Add up to 500 mL Total 2.5 M 500 mL Store this solution at room temperature.
40% PEG 8000/2.5 M NaCl
Reagent Final concentration Quantity 5 M NaCl (see Recipe 5) 2.5 M 500 mL PEG8000 40% 400 g ddH2O n/a Add up to 1,000 mL Total n/a 1,000 mL Prime a 0.22 µm pore size filter with 2.5 mL of 2.5 M NaCl before filtering the 40% PEG 8000/2.5 M NaCl solution. The filtering will take over an hour due to the viscosity of the solution. Store at room temperature.
TMN
Reagent Final concentration Quantity 1 M Tris-HCl pH 8.0 50 mM 50 mL 2 M MgCl2 5 mM 2.5 mL NaCl 150 mM 8.77 g ddH2O n/a Add up to 1,000 mL Total n/a 1,000 mL Adjust solution to pH 8.0. Filter with a 0.22 µm pore size filter. Store this solution at room temperature.
HBS
Reagent Final concentration Quantity HEPES 50 mM 2.980 g NaCl 150 mM 2.192 g Sarcosyl 1% 2.5 g EDTA 20 mM 1.46 g ddH2O n/a Add up to 250 mL Total n/a 250 mL Adjust solution to pH 8.0. Filter with a 0.22 µm pore size filter. Store this solution at room temperature.
5% sodium deoxycholate
Reagent Final concentration Quantity Sodium deoxycholate 5% 28.2 g ddH2O n/a Add up to 500 mL Total 5% 500 mL Filter with a 0.22 µm pore size filter. Store this solution at room temperature. Protect from light.
PBS-MK
Reagent Final concentration Quantity MgCl2 2.7 mM 263 mg KCl 2 mM 149.1 mg DPBS n/a Add up to 1,000 mL Total n/a 1,000 mL Filter with a 0.22 µm pore size filter. Store this solution at 2–4 °C.
PBS-NMK
Reagent Final concentration Quantity NaCl 1 M 29.2 g MgCl2 2.7 mM 131.5 mg KCl 2 m 74.55 mg DPBS n/a Add up to 500 mL Total n/a 500 mL Filter with a 0.22 µm pore size filter. Store this solution at 2–4 °C.
DPBS/0.001% Pluronic F-68
Reagent Final concentration Quantity 1× DPBS n/a 500 mL 100× Pluronic F-68 0.001% 50 µL Total n/a 500 mL Store this solution at 2–4 °C.
BalanCD HEK293 media—powder reconstituted
Reagent Final concentration Quantity BalanCD HEK293 powder n/a 21.32 g Sodium bicarbonate 2.20 g ddH2O n/a Add up to 1,000 mL Total n/a 1,000 mL Ensure that the pH of the solution is between 6.7 and 7.4 and the osmolality is between 280 and 320 mOsm/kg. Filter with a 0.22 µm pore size filter. Store this solution at 2–4 °C for up to one year.
Laboratory supplies
Pipet-X pipette controller (Rainin, catalog number: 17011733 or equivalent)
20 µL pipette (Rainin, catalog number: 17008650 or equivalent)
200 µL pipette (Rainin, catalog number: 17008652 or equivalent)
1000 µL pipette (Rainin, catalog number: 17008653 or equivalent)
Filtered 20 µL pipette tips (Rainin, catalog number: 30389274 or equivalent)
Filtered 200 µL pipette tips (Rainin, catalog number: 30389276 or equivalent)
Filtered 1000 µL pipette tips (Rainin, catalog number: 30389272 or equivalent)
125 mL baffled Erlenmeyer flasks (Sigma-Aldrich, catalog number: CLS431404-1EA)
250 mL baffled Erlenmeyer flasks (VWR, catalog number: 75993-572)
0.6 mL microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 05-408-120)
1.5 mL microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 05-408-129)
0.5 mL microcentrifuge tubes (Thermo Fisher Scientific, catalog number: 02-707-357)
Microtube racks (Thermo Fisher Scientific, catalog number: 22-313630)
Countess cell counting chamber slides (Thermo Fisher Scientific, catalog number: C10228)
24-well polystyrene microplates (Thermo Fisher Scientific, catalog number: 08-772-1)
15 mL polypropylene centrifuge tubes (VWR, catalog number: 89039-666)
15 mL steel wire racks (Thermo Fisher Scientific, catalog number: 3422306)
50 mL steel wire racks (Thermo Fisher Scientific, catalog number: FB147916A)
250 mL polypropylene centrifuge tubes (Thermo Fisher Scientific, catalog number: 05-538-53)
0.22 µm bottle top filters (Genesee Scientific, catalog number: 25-235)
10 mL serological pipettes (VWR, catalog number: 29443-047)
5 mL serological pipettes (VWR, catalog number: 29442-422)
Amicon centrifugal filter units (Sigma-Aldrich, catalog number: UFC910024)
5 mL sterile syringes (Thermo Fisher Scientific, catalog number: 14955458)
20 G sterile hypodermic needles (Thermo Fisher Scientific, catalog number: 14-817-209)
Thinwall polypropylene centrifuge tubes (Beckman Coulter, catalog number: 326823)
96-well qPCR plates (Genesee Scientific, catalog number: 27-105)
Strip caps for qPCR plate (VWR, catalog number: ST401425)
Fine-tipped markers (Thermo Fisher Scientific, catalog number: 19-166-600)
Alcohol wipes (Thermo Fisher Scientific, catalog number: 22-037790)
Equipment
Biosafety cabinet (NuAire LabGard, Class II, type A2, catalog number NU-540-400UB10 or equivalent)
CO2 resistant shaker platform (Thermo Scientific, catalog number: 88881103 or equivalent)
Agilent qPCR Machine (Agilent, model: MX30005P or equivalent)
Countess automated cell counter (Invitrogen, catalog number: C10281 or equivalent)
Reach-in CO2 incubator (Cell IQ, catalog number: MCO-80ICL-PA or equivalent)
Avanti J-15R benchtop centrifuge (Beckman Coulter, catalog number: B99517 or equivalent)
JS-4750 swinging-bucket rotor (Beckman Coulter, catalog number: B77580 or equivalent)
60 mm diameter bottle adaptors (Beckman Coulter, catalog number: 392079 or equivalent)
18 mm diameter tube adaptors (Beckman Coulter, catalog number: 359473 or equivalent)
Avanti JXN-26 centrifuge (Beckman Coulter, catalog number: B38619 or equivalent)
JA-25.50 fixed-angle rotor (Beckman Coulter, catalog number: 363058 or equivalent)
JS-5.3 swinging-bucket rotor (Beckman Coulter, catalog number: 368690 or equivalent)
Optima XPN-90 ultracentrifuge (Beckman Coulter, catalog number: A99842 or equivalent)
SW32Ti swinging-bucket rotor package (Beckman Coulter, catalog number: 369694 or equivalent)
-80 °C freezer (PHCbi, model: MDF-DU502VHA-PA)
Water bath (Thermo Fisher Scientific, catalog number: FSGPD10 or equivalent)
Microfuge 18 centrifuge (Beckman Coulter, catalog number: BE-M18C or equivalent)
Test tube rocker (Thermo Fisher Scientific, catalog number: 12-815-6Q or equivalent)
(Optional) Millipore Biopak Polisher (Sigma-Aldrich, catalog number: CDUFBI001)
(Optional) Millipak Express 20 filter (Sigma-Aldrich, catalog number: MPGP02001)
Software and datasets
MxPro—Mx3005P (version 4.10, 2/15/23)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Kulkarni, A. A., Seal, A. G., Sonnet, C. and Oka, K. (2024). Streamlined Adeno-Associated Virus Production Using Suspension HEK293T Cells. Bio-protocol 14(3): e4931. DOI: 10.21769/BioProtoc.4931.
分类
生物工程 > 生物医学工程
细胞生物学 > 细胞工程
微生物学 > 异源表达系统 > 腺相关病毒
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









