- EN - English
- CN - 中文
A Protocol for Custom Biomineralization of Enzymes in Metal–Organic Frameworks (MOFs)
金属有机框架 (MOFs) 中酶的定制生物矿化方案
(*contributed equally to this work) 发布: 2024年02月05日第14卷第3期 DOI: 10.21769/BioProtoc.4930 浏览次数: 1869
评审: Salim GasmiNeha NandwaniAnonymous reviewer(s)
Abstract
Enzyme immobilization offers a number of advantages that improve biocatalysis; however, finding a proper way to immobilize enzymes is often a challenging task. Implanting enzymes in metal–organic frameworks (MOFs) via co-crystallization, also known as biomineralization, provides enhanced reusability and stability with minimal perturbation and substrate selectivity to the enzyme. Currently, there are limited metal–ligand combinations with a proper protocol guiding the experimental procedures. We have recently explored 10 combinations that allow custom immobilization of enzymes according to enzyme stability and activity in different metals/ligands. Here, as a follow-up of that work, we present a protocol for how to carry out custom immobilization of enzymes using the available combinations of metal ions and ligands. Detailed procedures to prepare metal ions, ligands, and enzymes for their co-crystallization, together with characterization and assessment, are discussed. Precautions for each experimental step and result analysis are highlighted as well. This protocol is important for enzyme immobilization in various research and industrial fields.
Key features
• A wide selection of metal ions and ligands allows for the immobilization of enzymes in metal–organic frameworks (MOFs) via co-crystallization.
• Step-by-step enzyme immobilization procedure via co-crystallization of metal ions, organic linkers, and enzymes.
• Practical considerations and experimental conditions to synthesize the enzyme@MOF biocomposites are discussed.
• The demonstrated method can be generalized to immobilize other enzymes and find other metal ion/ligand combinations to form MOFs in water and host enzymes.
Graphical overview
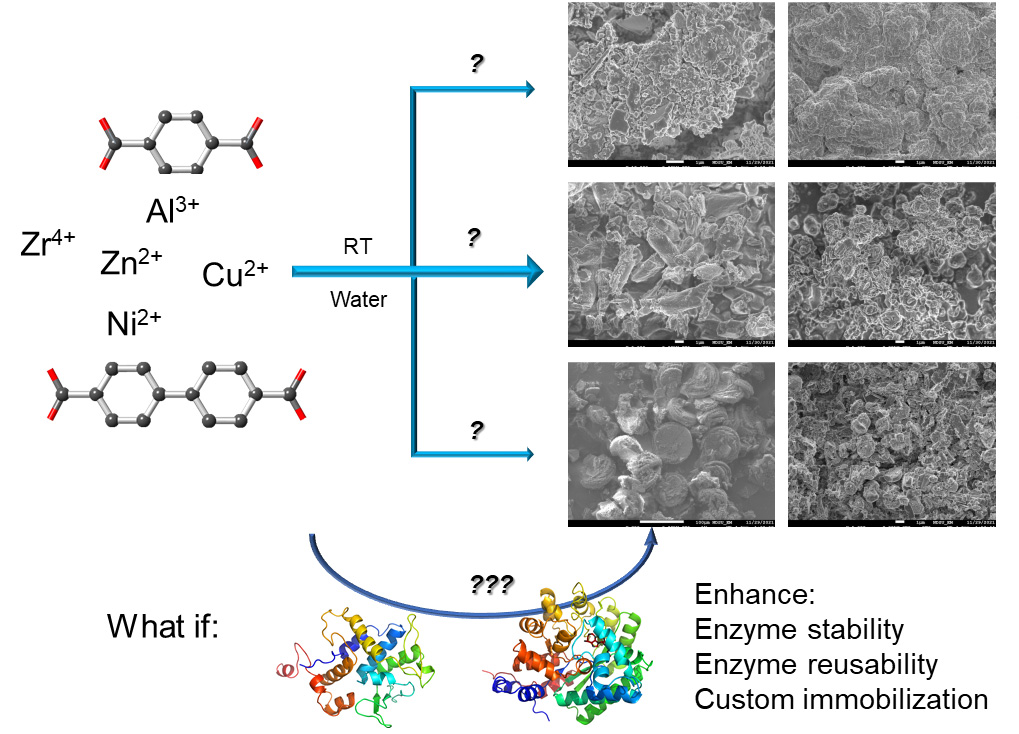
Background
Enzyme immobilization is receiving increased interest in both research and industry due to the (potential) promise of enhanced cost efficiency and catalytic performance control [1–4]. The biggest challenge is still maintaining enzymatic function without disturbance to the enzyme itself [5,6]. Metal–organic frameworks (MOFs) are extended 3-dimensional crystalline networks formed by the coordination bonds between certain metal ions and ligands. MOFs typically contain supreme porosity and highly tunable properties, given the high diversity in metal ions and ligands that can form such a 3D network. MOFs are advanced enzyme immobilization platforms but are mostly limited to smaller enzymes and substrates [6–13]. Co-crystallization of enzymes and certain metal ions/ligands is a unique way to host enzymes in MOF crystal scaffolds, being adaptable to large enzymes and enzyme clusters [14,15]. This strategy also allows for a small portion of enzymes to be implanted at the surface of MOF crystals, thus being partially exposed to the reaction medium, allowing contact with substrates larger than MOF apertures [16]. This strategy can therefore be applied to biocatalytic reactions—involving large substrates such as proteins/polypeptides, polysaccharides, and cells—to be carried out while reusing/recycling immobilized enzymes [17–25].
Commonly, the co-crystallization process is performed in an organic solvent such as methanol, which is a challenging condition for most enzymes [15]. There is a natural co-crystallization process called biomineralization, which is essentially co-crystallization but takes place in water phase under ambient conditions [26–28]. Although the reaction can be slow in nature, the formed biominerals are sufficiently stable with immobilized and possibly functional proteins/enzymes. Inspired by nature, biomineralization has also been carried out on lab benches [29,30]. Although nature might find its own sophisticated way to biomineralize proteins or other biomacromolecules, on-bench biomineralization in a reasonable timeframe for enzymes needs additional planning. Furthermore, although natural biomineralization could generate enzyme@MOF composites with distinct morphology and crystallinity, certain considerations are needed to optimize the on-bench strategy in order to better preserve enzyme activity and reusability and the stability of co-crystals. Lastly, aqueous phase co-crystallization may generate imperfect crystals but retains enzyme activity and reusability, which would still improve biocatalysis and thus be worth pursuing. Focusing on aqueous phase co-crystallization, we have acquired extensive experience in immobilizing various enzymes on MOFs [16,20,21]. Our recent work has revealed a combination of 10 metal ions/ligands to carry out biomineralization for effective enzyme immobilization; broadening the spectrum of available metal/ligand pairs allows for custom immobilization of enzymes according to their characteristics and stability (in certain metal ions and ligands) [19]. However, there is a lack of detailed experimental protocols to carry out such a sophisticated mission.
In this protocol, we detail the biomineralization procedures, highlight the precautions, potential pitfalls, and suggested solutions, and summarize the assessment of successful enzyme biomineralization based on our recent experience. We will cover MOF preparation in aqueous phase (metal/ligand selection and preparation and criteria of a useful MOF) to obtain a background without enzymes, enzyme@MOF composite formation (enzyme preparation and activity assessment on MOFs), and additional experimental conditions that may help the formation of co-crystals without damaging the enzymes. The strategy and methods can be applicable to immobilizing other enzymes and searching for other metal ion/ligand combinations for custom immobilization of other enzymes.
Materials and reagents
Reagents
Terephthalic acid (BDC, 98%) (Millipore Sigma, catalog number: 185361-100G)
4,4’-Biphenyldicarboxylic acid (BPDC) (CHEM-IMPEX INT’L Inc., catalog number: 26841)
Bicinchoninic acid (BCA) kit for protein determination (Millipore Sigma, catalog number: BCA1)
MES (Millipore Sigma, catalog number: 475893-100GM)
Sodium acetate (Millipore Sigma, catalog number: S8750)
HEPES (Millipore Sigma, catalog number: PHG0001-100G)
Glycine (Millipore Sigma, catalog number: 410225)
Ethanol (EtOH) (standard resources—could be obtained from any commercial resources)
Acetic acid (standard resources)
Sodium hydroxide (NaOH) (standard resources)
Concentrated hydrochloric acid (HCl) (standard resources)
Double-distilled water (standard resources)
Thin test tubes (Wiretrol® II, catalog number: 5-000-2020)
Solutions
MES buffer 0.5 M, pH 6 (10 mL) (see Recipes)
Acetate buffer 0.05 M, pH 4.6 (40 mL) (see Recipes)
HEPES buffer 0.2 M, pH 6.8 (40 mL) (see Recipes)
Glycine buffer 0.05 M, pH 9 (40 mL) (see Recipes)
Recipes
MES buffer 0.5 M, pH 6 (10 mL)
8 mL of double-deionized water (ddH2O)
0.976 g of MES
pH to 6 using HCl
Dilute to 10 mL
Acetate buffer 0.05 M, pH 4.6 (40 mL)
30 mL of ddH2O
82.272 mg of sodium acetate
0.057 mL of glacial acetic acid
pH to 4.6 using HCl
Dilute to 40 mL
HEPES buffer 0.2 M, pH 6.8 (40 mL)
30 mL of ddH2O
1.91 g of HEPES
pH to 6.8 using HCl
Dilute to 40 mL
Glycine buffer 0.05 M, pH 9 (40 mL)
30 mL of ddH2O
150 mg of glycine
10 mg of sodium hydroxide
pH to 9 with NaOH
Dilute to 40 mL
Equipment
Centrifuge (Thermo Fisher, model: Sorvall Legend Micro 21R)
Thermogravimetric analyzer (TA instruments Water, model: TGA-Q500)
XRD–Brucker’s Single Crystal Diffractometer, Apex 2 Duo with Cu IμS X-ray Source (Bruker Corporation, model: Apex 2 Duo)
Field-emission scanning electron microscope (STEM, model: JEOL JSM-7600F)
Millipore concentrators (Merck KGaA, catalog number: UFC500396)
Mortar and pestle setup (Millipore Sigma, catalog number: Z136077)
Spectrometer (Thermo Scientific, model: NanoDrop 2000)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Armstrong, Z., Jordahl, D., MacRae, A., Li, Q., Lenertz, M., Shen, P., Botserovska, A., Feng, L., Ugrinov, A. and Yang, Z. (2024). A Protocol for Custom Biomineralization of Enzymes in Metal–Organic Frameworks (MOFs). Bio-protocol 14(3): e4930. DOI: 10.21769/BioProtoc.4930.
分类
生物工程 > 生物医学工程
生物化学 > 其它化合物 > 离子
生物化学 > 蛋白质 > 活性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link