- EN - English
- CN - 中文
Mesenteric Parametrial Fat Pad Surgery for in vivo Implantation of Hepatocytes in Nude Mice
肠系膜旁脂肪垫手术在裸鼠体内植入肝细胞
(*contributed equally to this work) 发布: 2024年01月20日第14卷第2期 DOI: 10.21769/BioProtoc.4925 浏览次数: 1950
评审: Pilar Villacampa AlcubierreAras MattisWilliam C. W. Chen
Abstract
Cell-based liver therapies utilizing functionally stabilized engineered hepatic tissue hold promise in improving host liver functions and are emerging as a potential alternative to whole-organ transplantation. Owing to the ability to accommodate a large ex vivo engineered hepatocyte mass and dense vascularization, the mesenteric parametrial fat pad in female nude mice forms an ideal anatomic microenvironment for ectopic hepatocyte transplantation. However, the lack of any reported protocol detailing the presurgical preparation and construction of the engineered hepatic hydrogel, fat pad surgery, and postsurgical care and bioluminescence imaging to confirm in vivo hepatocyte implantation makes it challenging to reliably perform and test engraftment and integration with the host. In this report, we provide a step-by-step protocol for in vivo hepatocyte implantation, including preparation of hepatic tissue for implantation, the surgery process, and bioluminescence imaging to assess survival of functional hepatocytes. This will be a valuable protocol for researchers in the fields of tissue engineering, transplantation, and regenerative medicine.
Key features
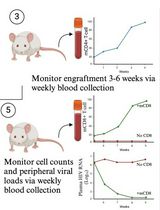
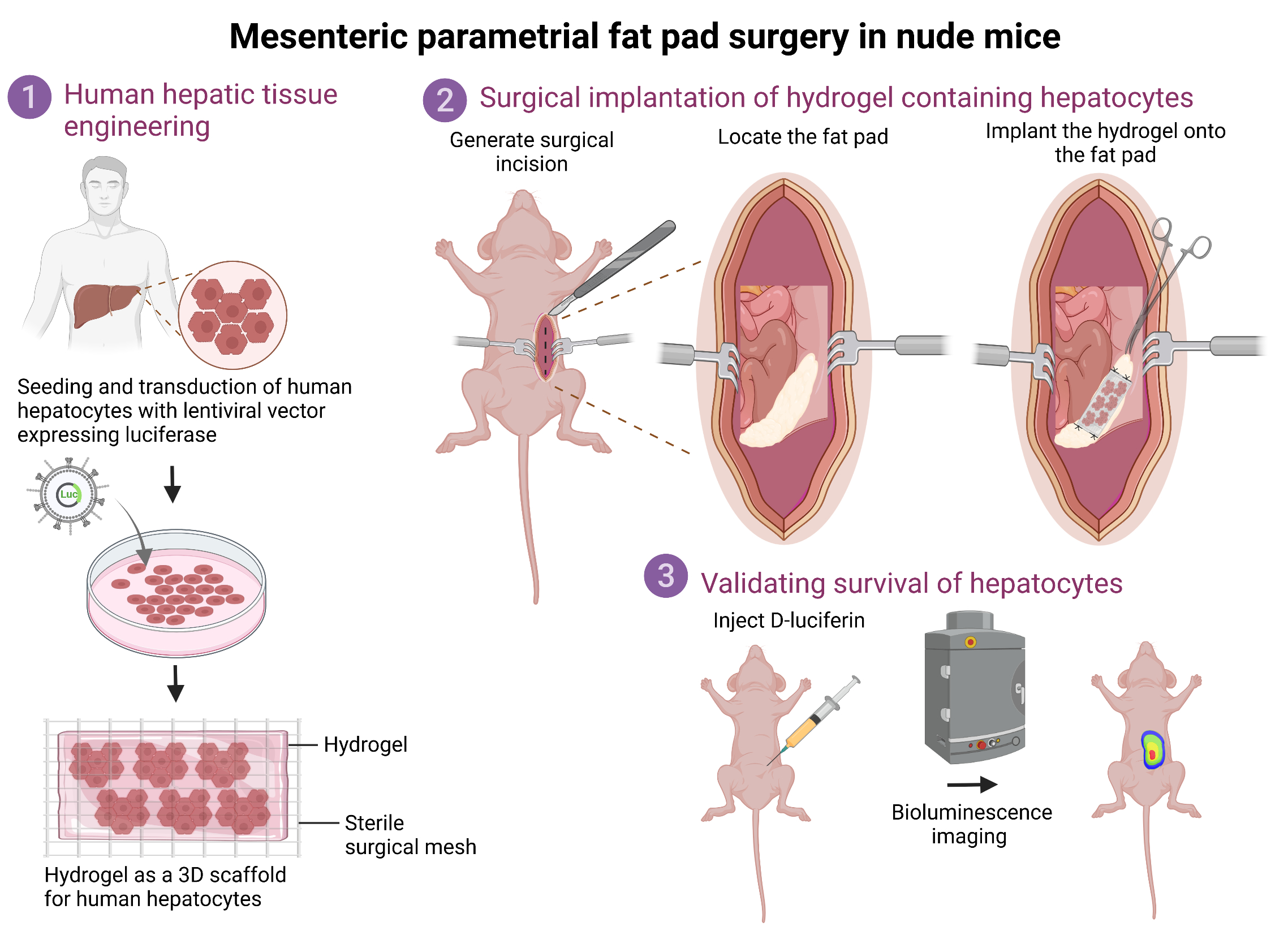
• Primary human hepatocytes transduced ex vivo with a lentiviral vector carrying firefly luciferase are surgically implanted onto the fat pad.
• Bioluminescence helps monitor survival of transplanted hepatic tissue over time.
• Applicable for assessment of graft survival, graft-host integration, and liver regeneration.
Graphical overview
Background
The liver is the largest and an essential internal organ of the human body, as it performs a multitude of detoxification, metabolic, synthetic, and immunologic functions. Most importantly, the liver possesses a unique ability to spontaneously regenerate after being subjected to damaging insults or injuries. Owing to this regenerative potential, orthotopic liver transplantation offers a valuable treatment option for patients suffering from end-stage liver diseases [1]. However, this is the only curative treatment option and remains limited due to organ scarcity and to patients who are not at a very advanced age, do not have any significant comorbidities, and are able to undergo a surgical procedure. Additionally, financial challenges and the long-term immunosuppression required posttransplant often make liver transplantation a last resort. Given the current organ shortage and paucity of living donors, it is becoming increasingly challenging to tackle the burden of liver diseases such as nonalcoholic fatty liver disease, cirrhosis, and hepatocellular carcinoma, which account for ~2 million deaths per year worldwide [2]. Hence, cell-based therapies offer new therapeutic options to restore liver function in patients with chronic liver diseases [3].
The current protocol describes the detailed procedures to perform ectopic transplantation of human hepatic tissue onto the mesenteric parametrial fat pad in an immune-compromised mouse model. Specifically, in this protocol, primary human hepatocytes are transduced with a bioluminescent lentiviral vector and plated in 3D coculture with mouse fibroblasts to form hepatic aggregates. These aggregates are embedded inside a hydrogel to form a hepatic tissue, which is subsequently transplanted onto the mesenteric parametrial fat pad in nude mice. Bioluminescent signal from transplanted hepatic tissue allows monitoring of the tissue over time. This valuable ectopic human hepatocyte transplantation model can be used for understanding important aspects of liver tissue engineering such as graft survival, graft-host integration, and liver regeneration.
Materials and reagents
Biological materials
Plateable cryopreserved primary human hepatocytes (Lonza, catalog number: HUCPG)
Mouse 3T3-J2 fibroblasts (Kerafast, catalog number: EF3003)
Female nude mice, 8–10 weeks (Taconic Biosciences, catalog number: NCRNU)
Reagents
Reagents required for tissue culture
Fibrinogen from bovine plasma (Millipore Sigma, catalog number: F8630)
Thrombin from bovine plasma (Millipore Sigma, catalog number: T4648)
AggreWellTM 400 microwell plates (STEMCELL Technologies, catalog number: 34411)
DMEM, high glucose, pyruvate (Thermo Fisher Scientific, catalog number: 11995065)
Fetal bovine serum (FBS), premium select, heat inactivated (R&D Systems, catalog number: S11550H)
Penicillin-Streptomycin (10,000 U/mL) (Thermo Fisher Scientific, catalog number: 15140122)
CorningTM ITS+ premix universal culture supplement (Fisher Scientific, catalog number: CB-40352)
HEPES (1 M) (Thermo Fisher Scientific, catalog number: 15630080)
Dexamethasone (Millipore Sigma, catalog number: D4902)
Glucagon (Millipore Sigma, catalog number: G2044)
Polybrene, 10 mg/mL, liquid (EMD Millipore, catalog number: TR-1003-G)
CorningTM HepGoTM hepatocyte culture medium (Fisher Scientific, catalog number: MT454670)
Lentiviral vector expressing firefly luciferase under the human albumin promoter (pTRIP.Alb.IVSb.IRES.tagRFP-DEST) (a gift from Charles Rice, The Rockefeller University, New York)
Trypan Blue solution, 0.4%, sterile filtered, suitable for cell culture (Sigma-Aldrich, catalog number: T8154)
Reagents required for surgery
Isoflurane, 3% (vol/vol) (Covetrus, catalog number: 029405)
Puralube vet ointment (Patterson Veterinary, catalog number: 07-888-2572)
Betadine solution 10% (vol/vol) povidone-iodine (Amazon, model number: 104457)
0.25% bupivacaine hydrochloride injection, 2.5 mg/mL (Covetrus, catalog number: 061842)
Meloxicam injectable solution, 5 mg/mL (Covetrus, catalog number: 049755)
Phosphate-buffered saline (PBS) (10×) pH 7.4, RNase-free, 0.9% (wt/vol) (Fisher Scientific, catalog number: AM9625)
Dietgel recovery (ClearH2O, catalog number: 72-06-5022)
D-Luciferin, potassium salt (GoldBio, catalog number: 115144-35-9)
Prescription-only medicines
Ethiqa XR (buprenorphine) extended-release injectable suspension, 1.3 mg/mL, C3 (Covetrus, catalog number: 072117)
Solutions
1× Phosphate-buffered saline solution (see Recipes)
Hepatocyte culture media (see Recipes)
50 mg/mL fibrinogen solution (see Recipes)
200 U/mL thrombin solution (see Recipes)
15 mg/mL D-Luciferin solution (see Recipes)
Recipes
1× Phosphate-buffered saline (PBS) solution
100 mL of PBS (10×) pH 7.4, RNase-free, 0.9% (wt/vol)
900 mL of distilled H2O
Hepatocyte culture media
432 mL of DMEM
50 mL of FBS
5 mL of Penicillin-Streptomycin
5 mL of ITS premix universal culture supplement
7.5 mL of HEPES (1 M)
0.4 µg/mL Dexamethasone
7 ng/mL Glucagon
50 mg/mL fibrinogen solution
1 g of fibrinogen from bovine plasma
20 mL of 1× PBS
200 U/mL thrombin solution
1,000 U thrombin from bovine plasma
5 mL of 1× PBS
15 mg/mL D-Luciferin solution
Reconstitute sterile D-luciferin powder to a concentration of 15 mg/mL in sterile 1× PBS. Aliquot and store at -20 °C. Thaw at room temperature prior to injection.
Laboratory supplies
CytoOne 35 × 10 mm TC dish (USA Scientific Inc, catalog number: CC76823340)
1.7 mL microcentrifuge tubes, PP (VWR, catalog number: 87003-294)
M-fold paper towels (McKesson, catalog number: 858231)
Equipment
Equipment for tissue culture
CellGard® ES Class II type A2 biological safety cabinet (NuAire, Inc., model: NU-475)
HeracellTM 150i CO2 incubator (Thermo Fisher Scientific, catalog number: 50116047)
Equipment for surgery
Dual procedure circuit/anesthesia chamber (VetEquip Inc., catalog number: 921400)
VaporGuard activated charcoal filter (VetEquip Inc., catalog number: NC9270393)
Germinator 500, dry sterilizer (Braintree Scientific, Inc., catalog number: GER 5287-120V)
Gel heating pad (Fisher Scientific, catalog number: 14-370-223)
Round hood to scavenge waste anesthetic vapors (Alsident System, catalog number: 1-7528)
Surgical light source (Stryker Berchtold Chromophare F 300)
Stainless steel digital animal weighing scale (Kent Scientific Corporation, catalog number: SCL-4000)
Towel drape, sterile, 18 × 25 (Dynarex, catalog number: 4410)
Alcohol preps, medium, 2-ply (Webcol, catalog number: 6818)
Self-seal sterilization pouches (VWR, catalog number: 89140-800)
PI polyisoprene surgical gloves, sterile (Protexis, catalog number: 2D72PT80X)
Transparent surgical tape (MD Supplies, catalog number: MPR-62201)
Inguinal hernia repair mesh Bard® flat sheet nonabsorbable polypropylene monofilament 3 × 6 in. rectangle style white sterile (McKesson, catalog number: 413782)
Sterile cotton-tipped wood applicator sticks (Patterson Veterinary, catalog number: 07-847-3562)
1 mL insulin syringes (Fisher Scientific, catalog number: 14-841-31)
Surgical scissors, sharp (Fine Science Tools, catalog number: 14002-13)
Graefe forceps (Fine Science Tools, catalog number: 110049-10)
Flat, round tip tweezers (Electron Microscopy Sciences, catalog number: 78333-33A)
Colibri retractor (Fine Science Tools, catalog number: 17000-03)
Hemostat (Fine Science Tools, catalog number: 13008-12)
Monocryl (poliglecaprone 25) suture (Ethicon, catalog number: Y303H)
Reflex wound clips (7 mm) (Fine Science Tools, catalog number: 12032-07)
Reflex clip removing forceps (World Precision Instruments, catalog number: 500347)
IVIS spectrum CT in vivo imaging system (Perkin Elmer, catalog number: 128201)
Software and datasets
Living Image 3.2 software (IVIS Imaging Systems)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sinha, S., Nguyen, D. T., Hassan, N., Ali, Q., Sethiadi, J., Tsoy, S. and Schwartz, R. E. (2024). Mesenteric Parametrial Fat Pad Surgery for in vivo Implantation of Hepatocytes in Nude Mice. Bio-protocol 14(2): e4925. DOI: 10.21769/BioProtoc.4925.
- Stevens, K. R., Scull, M. A., Ramanan, V., Fortin, C. L., Chaturvedi, R. R., Knouse, K. A., Xiao, J. W., Fung, C., Mirabella, T., Chen, A. X., et al. (2017). In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease. Sci. Transl. Med. 9(399): eaah5505.
分类
生物工程 > 生物医学工程
细胞生物学 > 细胞移植 > 异种移植
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link