- EN - English
- CN - 中文
The Development of an Advanced Model for Multilayer Human Skin Reconstruction In Vivo
开发先进的多层人体活体皮肤重建模型
发布: 2024年01月20日第14卷第2期 DOI: 10.21769/BioProtoc.4919 浏览次数: 2720
评审: Pilar Villacampa AlcubierreEVANGELOS THEODOROUAnonymous reviewer(s)
Abstract
Human skin reconstruction on immune-deficient mice has become indispensable for in vivo studies performed in basic research and translational laboratories. Further advancements in making sustainable, prolonged skin equivalents to study new therapeutic interventions rely on reproducible models utilizing patient-derived cells and natural three-dimensional culture conditions mimicking the structure of living skin. Here, we present a novel step-by-step protocol for grafting human skin cells onto immunocompromised mice that requires low starting cell numbers, which is essential when primary patient cells are limited for modeling skin conditions. The core elements of our method are the sequential transplantation of fibroblasts followed by keratinocytes seeded into a fibrin-based hydrogel in a silicone chamber. We optimized the fibrin gel formulation, timing for gel polymerization in vivo, cell culture conditions, and seeding density to make a robust and efficient grafting protocol. Using this approach, we can successfully engraft as few as 1.0 × 106 fresh and 2.0 × 106 frozen-then-thawed keratinocytes per 1.4 cm2 of the wound area. Additionally, it was concluded that a successful layer-by-layer engraftment of skin cells in vivo could be obtained without labor-intensive and costly methodologies such as bioprinting or engineering complex skin equivalents.
Key features
• Expands upon the conventional skin chamber assay method (Wang et al., 2000) to generate high-quality skin grafts using a minimal number of cultured skin cells.
• The proposed approach allows the use of frozen-then-thawed keratinocytes and fibroblasts in surgical procedures.
• This system holds promise for evaluating the functionality of skin cells derived from induced pluripotent stem cells and replicating various skin phenotypes.
• The entire process, from thawing skin cells to establishing the graft, requires 54 days.
Graphical overview
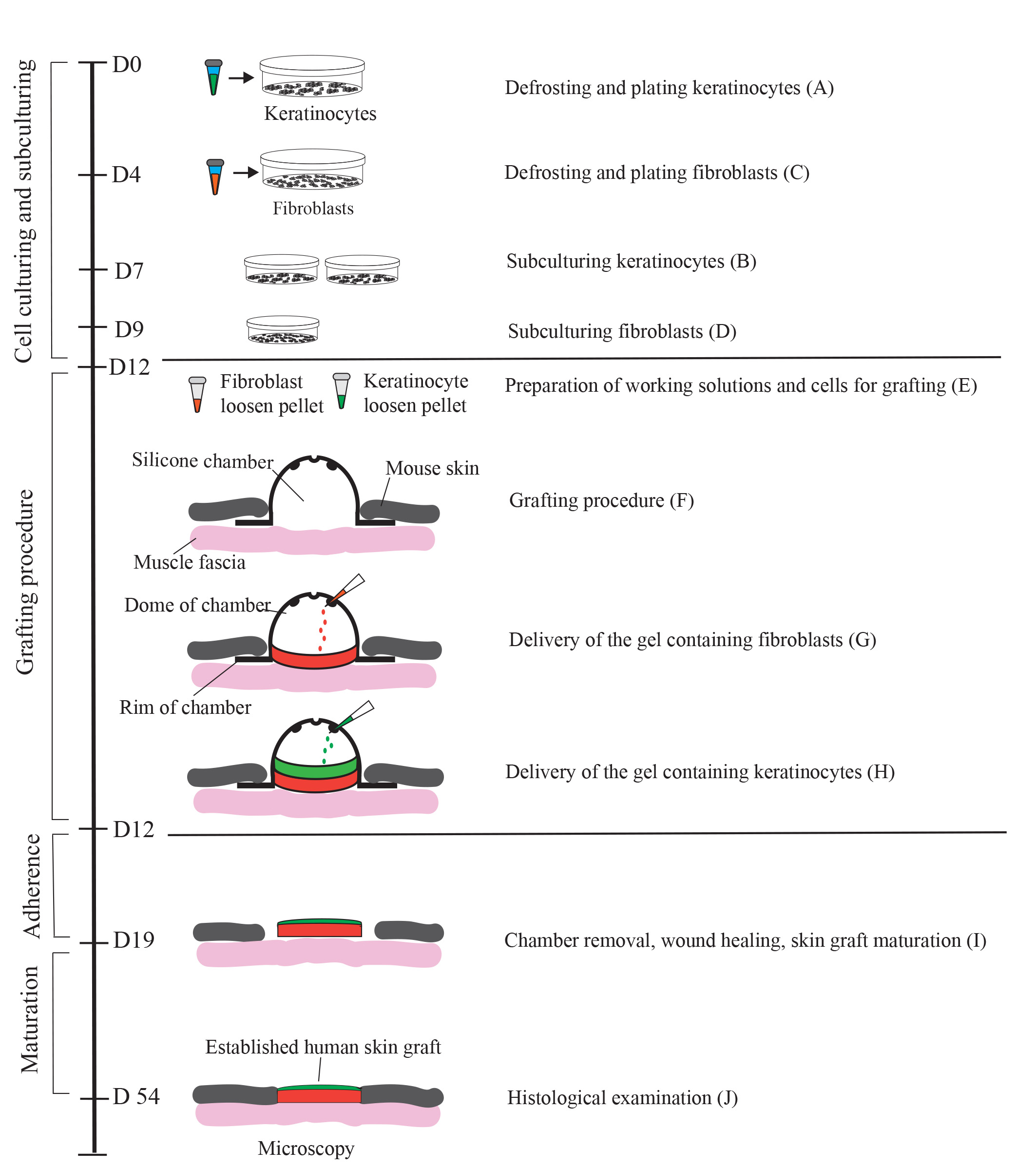
Generation of a human skin equivalent on an immunodeficient mouse using a fibrin-based grafting system. A schematic of the protocol is shown. Cultured keratinocytes and fibroblasts resuspended in a fibrin-based gel are delivered as layers into a silicon chamber inserted underneath the skin of an immunocompromised mouse. First, a fibrin gel containing encapsulated fibroblasts (up to 2 × 106 per 1.4 cm2 wound) is delivered into the chamber and allowed to solidify for 15 minutes. Second, a fibrin gel containing 1.0–2.0 × 106 keratinocytes is applied on top of the fibroblast layer. On day 7 post-grafting, the chamber is removed, and the wound with the graft is allowed to heal for 4–5 weeks. During healing, a scab forms and eventually falls off. By day 54, the graft is fully established.
Background
Progress in mouse genetics has provided a powerful research tool for elucidating the mechanisms of epidermal stem cell commitment and skin homeostasis. However, given the dramatic difference in skin structure between humans and mice, one must be cautious in applying the insights gained in studying mouse skin to human skin, especially when investigating skin diseases. For example, the inbred genetic background of mice can influence the phenotype resulting from disease-associated mutations. Humans are of mixed genetic background, and this complexity results in phenotypical variations and a different penetrance of genetically defined diseases, such as epidermolysis bullosa, a group of rare inherited skin blistering diseases. For this reason, developing complex in vivo human xenograft models is critical to advance our understanding of human skin conditions and developing new therapeutic interventions.
Several human xenograft models have been previously published, including a skin flap assay (Qiao et al., 2008), human skin transplantation-like approaches for grafting dermal-epidermal equivalents onto opened wounds of recipient mice (Escamez et al., 2004; Martinez-Santamaria et al., 2012; Yanez et al., 2015; Jorgensen et al., 2020), and chamber grafting assay (Wang et al., 2000; Diette et al., 2020). While promising, all these assays suffer from several limitations. They include (1) the complexity of the procedure that requires the generation of 3D skin equivalents in cell culture conditions before the transplantation onto a mouse, and (2) the limited availability and proliferative capacity of primary human cells, especially cells derived from patients with rare inherited skin diseases. A chamber grafting assay (Wang et al., 2000; Diette et al., 2020) provides a simplified procedure for grafting primary human skin cells since the assay promotes the self-assembly of fibroblasts and keratinocytes in an in vivo environment without the need to produce skin equivalents in a dish. However, successful production of human skin equivalents in the grafting assay still requires at least 5 × 106 primary keratinocytes per 1.4 cm2 of the wound area, which is a significant number when the availability of primary patient cells is limited. In addition, since this assay relies on the ability of keratinocytes and fibroblasts to self-assemble and form the dermis and epidermis, this limits the ability to incorporate other cell types that normally reside in the skin into an appropriate skin layer. Therefore, we aimed to modify the grafting chamber assay by decreasing the number of primary skin cells necessary for transplantation and mimicking the natural three-dimensional structure of the human skin in vivo to make a more viable in vivo model to study human skin conditions.
To achieve this, we modified the delivery of human keratinocytes and fibroblasts into the grafting chamber by first suspending these cells in a fibrin-based hydrogel. We then performed the sequential transplantation of fibroblasts followed by keratinocytes to recapitulate two major layers of the skin, the dermis and the epidermis, in a controlled manner. Due to its simplicity, this method can be easily optimized by incorporating additional cell types of different origins and ensuring that they are targeted explicitly into the dermis or the epidermis. In addition to grafting primary keratinocytes and fibroblasts, this system can be appropriate for testing the functionality of skin cells derived from induced pluripotent stem cells. The system is also applicable for testing novel skin transplantation systems for potential clinical applications, including cell harvesting and application techniques for treating cutaneous skin defects and pigmentation disorders.
Materials and reagents
Biological materials
Nude mice as recipients for grafting: homozygous nude Foxn1nu, formerly Hfh11nu, females, 6–8 weeks (Jackson Laboratory, Nu/J, #002019; https://www.jax.org/strain/002019). Alternatively, the homozygous NOD-Prkdcscid mice can be recipients for grafting (Jackson Laboratory, NOD.Cg-Prkdcscid/J, # 001303). Both nude and NOD-SCID mouse strains show efficient engraftment of human cells. However, NOD-SCID mice require depilation on the back before surgery and often before graft harvest. Therefore, careful consideration should be given to selecting the appropriate mouse strain for immunological studies to ensure compatibility with the experimental requirements and to minimize any potential confounding factors related to hair removal (Waldron-Lynch et al., 2012; Cristobal et al., 2021).
Note: Before preparing cell cultures, ensure that all mice are healthy and fully acclimated to the facility for at least a week. Ensure that the weight of the mice is above 21 g.
Fibroblasts, primary, neonatal (ATCC, catalog number: PCS-201-010)
Human epidermal keratinocytes, primary, neonatal (HEKn) (ThermoFisher, catalog number: C0015)
Reagents
DMEM/F12 (1:1) (ThermoFisher, catalog number 11320-033)
Pooled human AB serum derived (Innovative Research, ISERAB)
MEM-NEAA (ThermoFisher, catalog number: 11140050
GlutaMax (ThermoFisher, catalog number: 35050061)
2-Mercaptoethanol (ThermoFisher, catalog number: 21985023)
L-Ascorbic acid (Sigma-Aldrich, catalog number: A4544)
Hydrocortisone (Sigma-Aldrich, catalog number: H0888)
Antibiotic/Antimycotic (ThermoFisher, catalog number: 15240062)
Human bFGF (ThermoFisher, catalog number: PHG0263)
Human EGF (ThermoFisher, catalog number: PHG0313)
EpiLife medium (ThermoFisher, catalog number:MEPI500CA)
EpiLife defined growth supplement (EDGS) (ThermoFisher, catalog number: S0125)
Bovine collagen solution, type I, 3 mg/mL (Advanced BioMatrix, catalog number 5005).
Fetal bovine serum, qualified, one shot, raw (ThermoFisher, catalog number: A31605-01)
Aprotinin (Sigma-Aldrich, catalog number: A6279-10ML)
Fibrinogen from human plasma (Sigma-Aldrich, catalog number: F3879-1G)
Thrombin from human plasma (Sigma-Aldrich, catalog number: T4393-100UN)
DPBS without calcium and magnesium (ThermoFisher, catalog number: 14190-144)
0.25% Trypsin-EDTA (ThermoFisher, catalog number: 25200-056)
Accutase (StemCell, catalog number: 7920)
CRYO defined, animal component free freezing medium, 2× (ZenBio, catalog number: CNT-CRYO-50)
CryoStor CS10, 1× (StemCell, catalog number: 100-1061)
Anti-mouse keratin (K)1 antibody, Rabbit, 1:500 dilution (BioLegend, catalog number: 905602)
Anti-mouse/human K14 antibody, Chicken, 1:2,000 dilution (BioLegend, catalog number: 906004)
Anti-human Loricrin antibody, Rabbit, 1:2,000 dilution (Abcam, catalog number: 176322)
Anti-human Vimentin antibody, Mouse, 1:500 dilution (Abcam, catalog number: 16700)
Anti-Rabbit-488 secondary antibody (Invitrogen, catalog number: A11008)
Anti-Chicken-594 secondary antibody (Invitrogen, catalog number: A11042)
Anti Mouse-594 secondary antibody (Invitrogen, catalog number: 11032)
Formalin solution, neutral buffered, 10% (Sigma-Aldrich, catalog number: HT501128)
Solutions
Stock solutions
Thrombin stock solution (25 U/mL): made from powder (see Recipes)
Fibrinogen stock solution (40 mg/mL): made from powder (see Recipes)
Bovine collagen solution, type I (3 mg/mL): ready to use
Working solutions
Complete FEM medium (fibroblasts culturing) (see Recipes)
Complete EpiLife medium (keratinocytes culturing) (see Recipes)
Collagen solution (coating for keratinocyte culture) (see Recipes)
Fibrin gel containing fibroblasts (see Recipes)
Fibrin gel containing keratinocytes (see Recipes)
Recipes
Recipes for stock solutions
Thrombin stock solution
Working under aseptic conditions in a biosafety cabinet, reconstitute thrombin in sterile DPBS (without Ca2+ and Mg2+) at a concentration of 25 U/mL. Make an aliquot and freeze at -20 °C. The day before the surgery, defrost the thrombin solution overnight at 2–8 °C. Keep thrombin on ice before applying it for hydrogel preparation in the grafting procedure. The solution should be used promptly; however, it can be refrigerated at 2–8 °C for up to three days.
Fibrinogen stock solution
Working under aseptic conditions in a biosafety cabinet, reconstitute fibrinogen in sterile DPBS (without Ca2+ and Mg2+) at a concentration of 40 mg/mL stepwise. First, lay a thin layer of fibrinogen powder on top of warm (37 °C) DPBS and let it soak for 5 min at 37 °C; repeat the soaking procedure with the rest of the fibrinogen. Second, put the tube with the fibrinogen-saline solution in a water bath at 37 °C for 30–60 min and agitate the tube gently every 10 min. Ensure that there are no clumps of dry fibrinogen on the tube walls. Third, leave the tube on the shaker at 2–8 °C overnight to complete the preparation of the fibrinogen stock solution. If a significant number of undissolved clumps are left, repeat the 30-min incubation at 37 °C. Do not exceed 1 h of incubation at 37 °C. The fibrinogen-saline solution must not be vortexed. Fibrinogen should be filter-sterilized using a 0.22 μm syringe filter. Do not use vacuum filtration since this will lead to the breakdown of the molecule during the filtration. Make 50–100 µL aliquots and freeze at -20 °C.
Note: Alternatively, bovine fibrinogen and thrombin can be used.
Recipes for working solutions
Complete FEM medium (Table 1)
Prepare FEM medium by supplementing DMEM/F12 with 5% human serum, 0.5× MEM-NEAA, 0.5× GlutaMAXTM supplement, 55 µM GibcoTM 2-mercaptoethanol, 1× HyClone Antibiotic/Antimycotic, 50 µg/mL ascorbic acid, and 1 µg/mL hydrocortisone. Perform 0.22 µm sterile filtration and store at 4 °C for up to one month. Before application, add human bFGF to a final concentration of 12 ng/mL and human EGF to a final concentration of 5 ng/mL.
Table 1. Composition of complete FEM medium
Reagent Stock concentration Final concentration Amount DMEM/F12 (1:1) 92.763 mL Human serum 100% 5% 5 mL MEM-NEAA 100× 0.5× 0.5 mL GlutaMax Supplement 100× 0.5× 0.5 mL 2-Mercaptoethanol 55 mM 55 µM 0.1 mL Ascorbic acid 50 mg/mL 50 µg/mL 0.1 mL Hydrocortisone 5 mg/mL 1 µg/mL 0.02 mL Antibiotic/Antimycotic 100× 1× 1 mL 1. Sterilize using a 0.22 µm filter; 2. Add bFGF and EGF as shown below AFTER filtration Reagent Stock concentration Final concentration Amount bFGF 100 µg/mL 12 ng/mL 0.012 mL EGF 100 µg/mL 5 ng/mL 0.005 mL Total n/a n/a 0.005 mL Complete EpiLife medium (Table 2)
Prepare complete EpiLife medium by adding EpiLife Defined Growth Supplement (EDGS) following the manufacturer’s instruction. Add antibiotic/antimycotic.
Table 2. Composition of complete EpiLife Medium
Reagent Stock concentration Final concentration Amount EpiLife basal medium n/a n/a 500 mL EDGS 100× 1× 5 mL Antibiotic/Antimycotic 100× 1× 5 mL Total n/a n/a 510 mL Collagen solution
To prepare the collagen working solution for the coating of the tissue culture dishes, dilute the 3 mg/mL bovine collagen stock solution to a final working concentration of 30 µg/mL in 1× DPBS. Use fresh.
Fibrin gel containing fibroblasts (Table 3)
To prepare the fibrin gel containing fibroblasts, first make working Solution 1. To make working Solution 1, supplement DMEM/F12 1:1 with 1× Antibiotic/Antimycotic, 1% raw FBS, and aprotinin. Mix components in an Eppendorf tube and keep the tube on ice until ready to graft. Just before grafting, mix Solution 1 with fibroblasts and then add fibrinogen and thrombin. Mix components and use for grafting.
Critical: Do not add fibroblasts, fibrinogen, and thrombin into Solution 1 until ready to graft*.
Table 3. Composition of the gel containing fibroblasts
Reagent Stock concentration Final concentration Amount Solution 1 DMEM/F12 (1:1) n/a n/a 291.7 µL Antibiotic/Antimycotic 100× 1× 4 µL Raw FBS 100% 1% 4.1 µL Aprotinin (3-7 TIU/mg protein) n/a 23.5 µL Just before grafting, add fibroblasts, fibrinogen, and thrombin as shown below. Reagent Stock concentration Final concentration Amount Fibroblasts* n/a 2 × 106 50 µL Fibrinogen* 40 mg/mL 1.73 mg/mL 17.3 µL Thrombin* 25 U/mL 0.59 U/mL 9.4 µL Total n/a n/a 400 µL Fibrin gel containing keratinocytes (Table 4)
To prepare the fibrin gel containing keratinocytes, first make working Solution 2. To make a working Solution 2, supplement EpiLife medium with 1× Antibiotic/Antimycotic and aprotinin. Mix components in an Eppendorf tube and keep the tube on ice until ready to graft. Just before grafting, mix Solution 2 with keratinocytes and then add fibrinogen and thrombin. Mix components and use for grafting.
Critical: Do not add keratinocytes, fibrinogen, and thrombin into Solution 2 until ready to graft**.
Table 4. Composition of the gel containing keratinocytes
Reagent Stock concentration Final concentration Amount Solution 2 EpiLife n/a n/a 295.8 µL Antibiotic/Antimycotic 100× 1× 4 µL Aprotinin (3-7 TIU/mg protein) n/a 23.5 µL Just before grafting, add keratinocytes, fibrinogen, and thrombin as shown below. Reagent Stock concentration Final concentration Amount Keratinocytes** n/a 2 × 106 50 µL Fibrinogen** 40 mg/mL 1.73 mg/mL 17.3 µL Thrombin** 25 U/mL 0.59 U/mL 9.4 µL Total n/a n/a 400 µL
Note: For a large-scale experiment, the working hydrogel solutions for the dermal and epidermal layers can be prepared as a combined mix to facilitate the generation of multiple grafts simultaneously. To maximize efficiency, 2–4 grafts can be performed in a single experimental setup. Optional: recalculate the volumes for Solution 1 and Solution 2 based on the number of grafts.
Equipment
Silicone chambers [produced by Qure Medical, 1810 Renaissance Boulevard, Sturtevant, WI, 53177, (262) 417–1307, http://www.qure-med.com, according to the following dimensions: 12 mm inner diameter (ID), 20 mm outer diameter (OD), 10 mm tall (Part# 12–24, upper chamber)]. Chambers are made of Momentive LSR 2650, two-component liquid silicone rubber for injection molding processes (Figure 2A).
Note: The chambers are reusable and autoclavable. Manually punch three holes in the dome of the chamber using a 2 mm biopsy punch to allow for gas exchange during the time the chamber is in place under the skin.
Nikon Eclipse 90i + Photometrics Cool SNAP HQ2+ DS Fi1 (Nikon, model: Ti2-E)
Nikon eclipse Ti-U inverted microscope (Nikon, model: Ti-U)
CO2 incubator (ThermoFisher, model: HERA cell VIOS 160)
Straight forceps (VWR, catalog number: 82027-398)
Surgical scissors (VWR, catalog number: 82027-584)
Curved forceps (VWR, catalog number: 89259-946)
Pipettes P1000 (Gilson, catalog number: F144059M)
Pipettes P200 (Gilson, catalog number: F144058M)
Pipettes P20 (Gilson, catalog number: F144056M)
Sterile filtration system (Corning, catalog number: 431097)
-80 °C freezer (ThermoFisher, model: TSX Series with V-Drive)
Heating pad-T/Pump Professional (Gaymar, catalog number: TP700)
Heating pad-blanket (Cincinnati Sub-Zero, model: 274)
Liquid bandage New skin (Amazon, catalog number: 851409007011)
Software and datasets
NIS-elements Nikon (https://www.nikoninstruments.com/Products/Software)
Microsoft Excel (Microsoft, https://products.office.com/en-us/excel)
GraphPad Prism (GraphPad, https://www.graphpad.com/scientific-software/prism/)
Adobe Illustrator (https://www.adobe.com/products/illustrator/free-trial-download.html)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Pavlova, M., Balaiya, V., Flores, J. C., Ferreyros, M., Bush, K., Hopkin, A., Kogut, I., Roop, D. R. and Bilousova, G. (2024). The Development of an Advanced Model for Multilayer Human Skin Reconstruction In Vivo. Bio-protocol 14(2): e4919. DOI: 10.21769/BioProtoc.4919.
分类
生物工程
细胞生物学 > 细胞移植 > 异种移植
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












