- EN - English
- CN - 中文
Live Imaging and Analysis of Meiotic Cytokinesis in Drosophila Testes
果蝇睾丸减数分裂胞质分裂的实时成像和分析
发布: 2024年01月20日第14卷第2期 DOI: 10.21769/BioProtoc.4918 浏览次数: 3039
评审: Giansanti MariagraziaPradeep Kumar BhaskarAnonymous reviewer(s)
Abstract
All living organisms require the division of a cell into daughter cells for their growth and maintenance. During cell division, both genetic and cytoplasmic contents are equally distributed between the two daughter cells. At the end of cell division, cytoplasmic contents and the plasma membrane are physically separated between the two daughter cells via a process known as cytokinesis. Hundreds of proteins and lipids involved in the cytokinetic process have been identified; however, much less is known about the mechanisms by which these molecules regulate cytokinesis, being therefore an intense area of current research. Male meiotic cytokinesis in Drosophila melanogaster testes has been shown to be an excellent model to study cytokinesis in vivo. Currently, several excellent protocols are available to study cytokinesis in Drosophila testes. However, improved methods are required to study cytokinesis under in vitro and ex vivo conditions. Here, we demonstrate a simple method to perform live imaging on individual spermatocyte cysts isolated from adult testes. We evaluate amenability of this in vitro method for treatment with pharmacological agents. We show that cytokinesis is strongly inhibited upon treatment with Dynasore, a dynamin inhibitor known to block clathrin-mediated endocytosis. In addition, we also demonstrate an ex vivo method to perform live imaging on whole mount adult testes on gas permeable membrane chambers. We believe the protocols described here are valuable tools to study cytokinetic mechanisms under various genetic and treatment conditions.
Key features
• In vitro method to study male meiotic cytokinesis in dissected spermatocyte cysts.
• In vitro method allows acute treatment with various pharmacological agents to study cytokinesis.
• Ex vivo method to image male meiosis cytokinesis in intact adult testes.
• Requires 15–60 min to set up and could be imaged up to 6–12 h.
Graphical overview
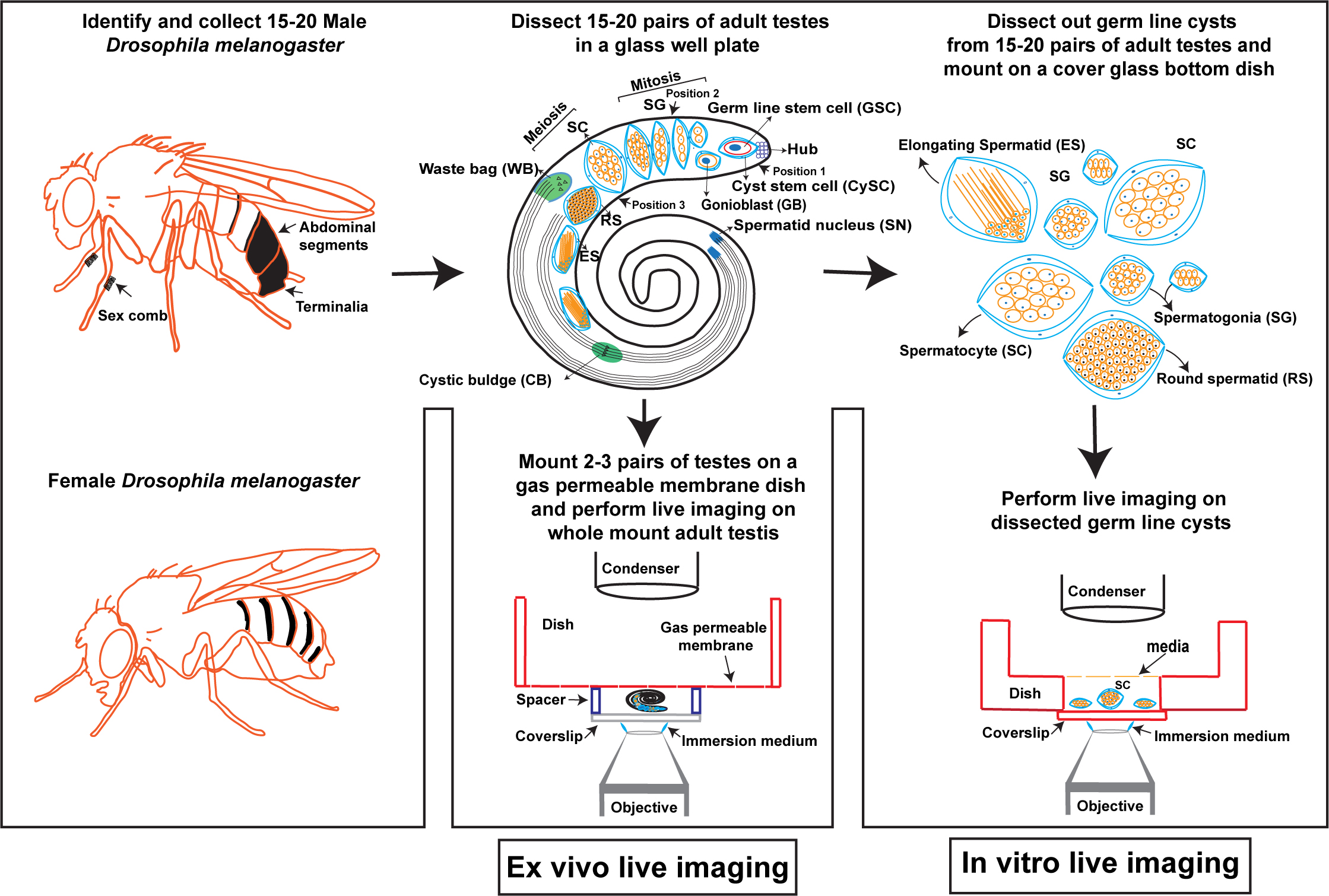
In vitro and ex vivo live imaging of male meiotic cytokinesis in adult Drosophila testes
Background
Cytokinesis is a final step of cell division wherein parent cell cytoplasmic contents and plasma membrane are physically divided between the two daughter cells. In animal cells, immediately after chromosomes are segregated to the poles, the spindle-mediated mechanisms determine the site of actomyosin ring assembly at the cell cortex. Subsequently, the actomyosin contractile ring that anchors the plasma membrane, and the central spindle begins to constrict, leading to the generation of cleavage furrow (Figure 1B). In mitotic cells, the plasma membrane between two daughter cells is pinched off in a process known as abscission, leading to the physical separation of daughter cells. However, unlike mitotic cells, in male germ cells abscission does not occur at the end of cytokinesis. As a result, all the daughter cells originating from the gonioblast are interconnected via their cytoplasmic bridges, known as ring canals. It is thought that the ring canals play an important role in intercellular communication and signaling [1]. All the cells in a cyst connected via cytoplasmic bridges undergo synchronous divisions [2, 3] (Video 1).
Video 1. In vitro live cell imaging of isolated adult Drosophila spermatocyte cyst undergoing meiosis I and meiosis II cytokinesis. The cysts are isolated from fly testes expressing GFP-tagged PH domain of Grp1/Step protein under the control of tubulin 84B promoter (tGPH) and mCherry tagged fascetto (feo) protein under the control of Ubi-p63E promoter (Feo-mCherry). The GFP-PH-Grp1/Step localizes to cytosol, plasma membrane, and cleavage furrow in cysts with intact cyst cell membrane. However, tGPH localizes only to the cytosol in cysts that have lost cyst cell membrane envelop. Feo-mCherry localizes to central spindle, independent of cyst cell membrane envelop. Live imaging was performed with the 63× water immersion objective and a pre-defined area of interest (AOI) size of 1024 × 1024 and at intervals of 2 min for 120 repeats. Video shown here represents 10 frames/second.
Drosophila melanogaster has been shown to be a valuable genetic model to study cytokinesis in vivo. Conventionally, Drosophila embryos undergoing cellularization [4, 5], proliferating larval neuroblasts [6, 7], and male meiotic spermatocytes [8, 9] have been used in cytokinesis studies. Meiotic spermatocytes in Drosophila testes offer several unique advantages over other systems to study cytokinesis. These include the relatively large size of meiotic spindle, weak spindle checkpoint, unambiguous identification of cytokinetic mutants due to characteristic morphological differences, and finally, live imaging of spermatocytes in isolated cysts or intact pupal testes. The relatively large size of male meiotic spindle is a favorable cytological feature in spermatocytes that allows precise localization of many cytokinetic proteins including microtubule and contractile ring–associated proteins. Male meiotic cells also show prominent central spindle (Figure 1B) (a bundle of antiparallel, interdigitating microtubules localizes to the center of segregating chromosomes where the assembly of contractile ring begins) [10, 11]. In neuroblasts, the presence of an abnormal spindle activates spindle checkpoint, which in turn arrests the cells in metaphase and precludes the observation of subsequent steps in cell division including cytokinesis [11–14]. However, due to weak spindle checkpoint, spermatocytes continue through the cell division stages despite the abnormal spindle. Thus, using meiotic cytokinesis, it is possible to ask whether a gene involved in spindle formation also has a role in later stages of cell division including cytokinesis. For instance, it was shown that mutations in the polo and abnormal spindle (asp) genes cause metaphase arrest in larval neuroblasts but produce frequent cytokinetic failures in spermatocytes [12–14]. Drosophila male meiosis is also an excellent model for cytokinesis due to the unique organization of mitochondria in round spermatids. During male meiotic cytokinesis, mitochondria is equally partitioned between the two daughter cells; immediately after cytokinesis, it aggregates to form a phase-dark structure known as Nebenkern. Failure in cytokinesis abrogates mitochondrial segregation; as a result, round spermatids show unusually large mitochondria associated with multiple nucleus (Figure S1) [15]. Thus, the presence of unusually large mitochondria with multiple nucleus is a characteristic feature of meiotic cytokinesis in spermatocytes (Figure S1) [3, 15].
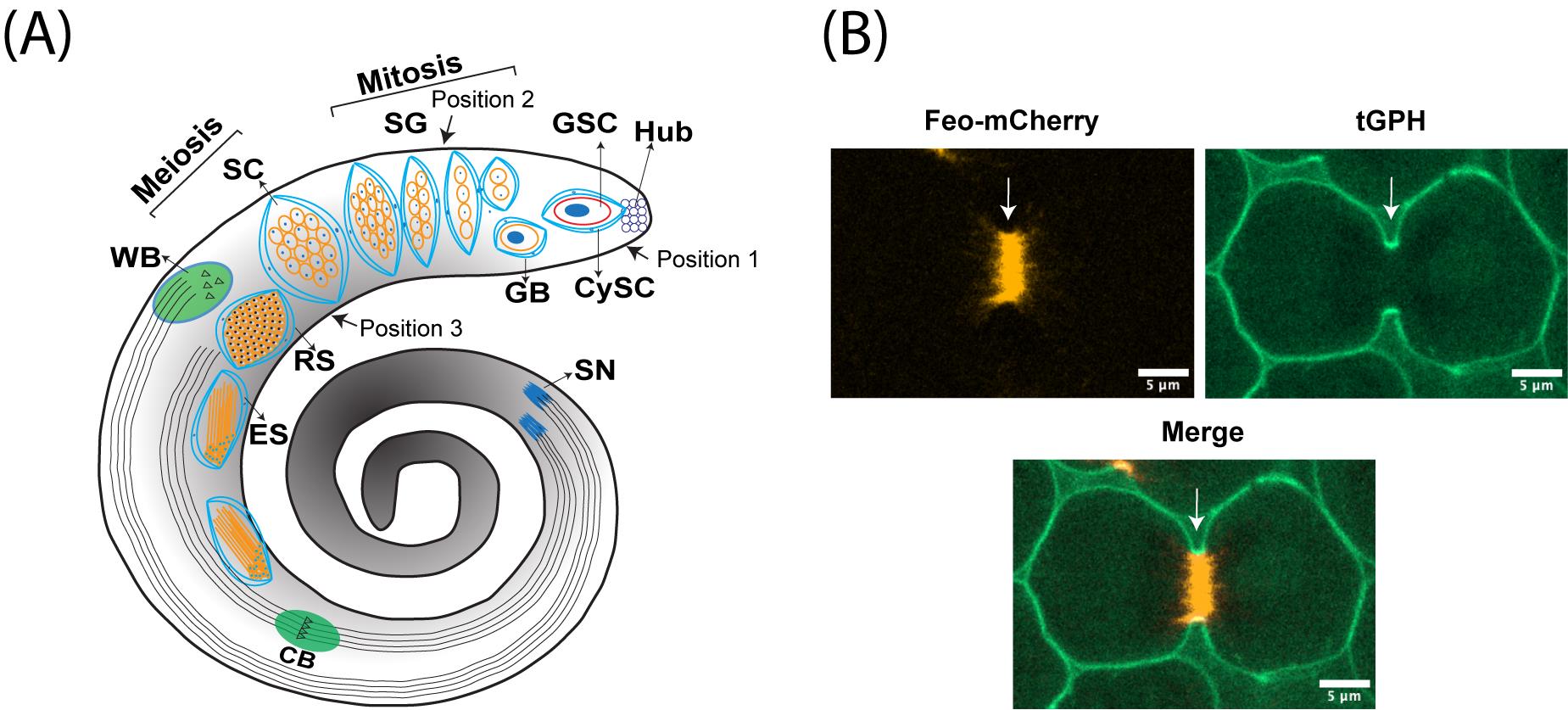
Figure 1. Male meiotic cytokinesis in adult Drosophila testis. (A) Cartoon representing dissected adult testis showing various stages of germ cell differentiation. Drosophila spermatogenesis shares a significant amount of similarity with the mammalian system. In many species, including Drosophila melanogaster, male germ cell differentiation is a sequential process. It begins with the germline stem cell (GSC) attached to the hub cells at the tip of the testis undergoing asymmetric division to yield one committed progenitor called gonioblast (GB). Two cyst stem cells (CySCs) that surround the GSC also undergo asymmetric division to produce two cyst cells that encase the GB. Subsequently, the GB undergoes four rounds of mitotic divisions, resulting in the 16-cell stage known as spermatogonia (SG). With the premeiotic S phase and an impressive growth during G2 phase due to robust transcriptional activity, spermatogonia become spermatocytes (SC), which in turn synchronously undergo two rounds of meiotic divisions (meiosis I & meiosis II) resulting in the haploid, 64-cell stage known as round spermatids (RS). Due to a lack of abscission step at the end of cytokinesis in germ cells, all cell divisions originating from a single GB are connected to each other via cytoplasmic bridges known as ring canals (RCs). After completion of meiosis, all the mitochondria in individual spermatids aggregate around the basal body at one end of the nucleus to form a unique structure called nebenkern. Subsequently, round spermatids elongate via complex morphological changes including polarization of spermatid nucleus, dramatic nuclear size reduction due to histamine to protamine switch, elongation of nebenkern, axoneme growth, and formation of acrosome, a membrane-bound organelle required for fertilization. Finally, the individual sperms are physically separated by a complex process known as individualization, wherein actin-based cones migrate from head to tail fashion with the concomitant removal of cytoplasmic contents [cystic bulge (CB) and waste bag (WB)] and wrapping each spermatozoon with its own plasma membrane. (B) Representative live images showing localization of central spindle–associated protein feo (fascetto tagged with mCherry) (left panel) and a plasma membrane–associated protein tGPH (PH domain of Grp1fused to GFP) (middle panel), during cytokinesis. Right panel shows the merge of both channels and arrow indicates the site of cleavage furrow.
Several excellent protocols have been described in the literature for the isolation of Drosophila testes from embryo to adult developmental stages and ex vivo imaging of whole mount pupal testes and isolated spermatocyte cysts [16–22]. However, detailed protocols focused on live imaging of male meiotic cytokinesis in dissected germ line cysts have not been described. Further, live imaging of male meiotic cytokinesis in whole mount adult testes has not been demonstrated. The protocols described here build upon the methods developed by Gartner et al. [18] and Karabasheva, G. and Smyth, J.T. [19], extending their application to male meiotic cytokinesis in adult Drosophila testes. Gartner et al. have standardized a method for live imaging of post-meiotic histone to protamine switch in intact pupal testes and dissected pupal germ line cysts [18]. Karabasheva, G. and Smyth J.T. established a method for live imaging of male meiotic cytokinesis in intact pupal testes [19]. Here, we demonstrate both in vitro and ex vivo methods to image male meiotic cytokinesis in adult testes. The in vitro method involves isolation of male germline cysts and imaging in a cover glass bottom dish. This in vitro method is of particular interest when the experimental settings require direct treatment of spermatocytes with external agents such as proteins, lipids, and pharmacological agents. Using this method, we show that treatment of spermatocytes with Dynasore, a dynamin inhibitor known to block clathrin-mediated endocytosis [23], also blocks male meiotic cytokinesis by preventing normal assembly of central spindle. The ex vivo method presented here involves dissection of adult testes and imaging on gas permeable membrane dish chambers. Both in vitro and ex vivo methods demonstrated here are extremely valuable to study male meiotic cytokinesis under various treatment conditions and genetic backgrounds.
Materials and reagents
Biological materials
Refer to Giansanti, G.M et al. [24], for a list of tagged proteins that can be used to mark central spindle/spindle midzone and cleavage furrow. For analysis of cytokinesis, a few examples of fly stocks are listed below (Table 1).
Table 1.List of fly stocks to label plasma membrane, central spindle, and actomyosin contractile ring .
| No | Protein | Localization | Fly stock number/reference |
|---|---|---|---|
| 1 | PH domain of Steppke/Grp1, expressed under the control of Tub84B promoter (tGPH), binds to PIP3 | Plasma membrane, enriched on early and late stage of cleavage furrow membranes | BDSC#8163 |
| 2 | Ubi-p63E-Feo-mCherry, mCherry-tagged feo protein is expressed under the control of ubiquitin regulatory sequence | Spindle midzone/central spindle | BDSC#59277 |
| 3 | Sqh-GFP.RLC, Myosin II regulatory light chain tagged to GFP is expressed under its own promoter control | Contractile ring | BDSC#57145 |
| 4 | UAS-mRFP-Scraps, PH domain containing Anillin protein tagged with mRFP expressed under UAS control | Cleavage furrow/contractile ring | BDSC#52220 |
| 5 | UAS-PLCdels-PH-EGFP, PH domain of human PLCD1 tagged with EGFP expressed under UAS control | Plasma membrane and cleavage furrow | BDSC#39693 |
Reagents
Shields and Sang M3 insect media (Sigma-Aldrich, catalog number: S8398-1L)
Heat-inactivated FBS suitable for insect cell culture (Thermo Fisher Scientific, catalog number: 10082147)
Penicillin-Streptomycin (10,000 U/mL) (Thermo Fisher Scientific, catalog number: 15140122)
Distilled water, 1,000 mL (Thermo Fisher Scientific, catalog number: 15230147)
Poly-D-Lysine hydrobromide (Sigma-Aldrich, catalog number: P0899)
Potassium bicarbonate (KHCO3) (Sigma-Aldrich, catalog number: 237205)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D2650)
Dynasore hydrate (Sigma-Aldrich, catalog number: D7693)
Ethanol (70 %)
M3 media (see Recipes)
Solutions
200 μg/mL Poly-D-Lysine hydrobromide in distilled water
31 mM or 10 mg/mL Dynasore hydrate in DMSO
Recipes
M3 media
Prepare this recipe in a biological safety cabinet. Dispense one bottle of powdered media (Shields and Sang M3 insect media) into a 1 L beaker (caution: may cause irritation to eyes and skin) and add 0.5 g of KHCO3 and 800 mL of sterile distilled water. Dissolve the powder completely using a magnetic stirrer and a bar. Subsequently, add 100 mL of heat-inactivated FBS and 10 mL of penicillin/streptomycin solution and mix well on a magnetic stirrer. Make up the final volume to 1 L and filter sterilize by passing through a bottle-top filter unit with 0.2 μm pore sized membrane. Aliquot the filter-sterilized media into 50 mL Falcon tubes and store at -20 °C. On the day of experiment, thaw one aliquot of media in a 37 °C water bath and filter the media again into a fresh Falcon tube using a 0.2 μm syringe filter. When performing the experiment, media should be at room temperature. Excess media can be stored at 4 °C for up to 1–2 weeks.
Laboratory supplies
Glass-bottom cell culture dish, 15 mm (NEST, catalog number: 801002)
Glass well plate, 9 wells (Corning Life Sciences, catalog number: 7220-85)
Dumont#5 ceramic-coated forceps (Fine Science Tools, catalog number: 11252-50)
Dissection pins, tip diameter 0.0175 mm (Fine Science Tools, catalog number: 26002-15)
Pin holders (Fine Science Tools, catalog number: 26018-17)
Lumox® dish 50 (Sarstedt, catalog number: 94.6007.410)
SecureSeal imaging Spacers, 0.12 mm depth (Grace Bio-Labs, catalog number: 654008)
Disposable sterile filter units, 500 mL (Thermo Fisher Scientific, catalog number: 450-0020)
Syringe filters, sterile, 0.22 um (GenClone®, catalog number: 25-244)
BD 10 mL syringe, luer-lok tip (BD, catalog number: 302995)
Microtubes, clear 1.7 mL (Olympus plastics, catalog number: 24-281)
Falcon, 50 mL, sterile, polypropylene conical tube, (Corning, catalog number: 352098)
15 mL centrifuge tube, sterile polypropylene tube (Corning, catalog number: 430052)
Equipment
Zeiss SteREO Discovery microscope (Zeiss Group, model: V12)
Spinning disk confocal on Leica DMi8 microscope base (Andor)
Software and datasets
Andor Fusion (version 2.3.0.44) (https://andor.oxinst.com/downloads/view/fusion-release-2.3)
Imaris (version 10.0) (https://imaris.oxinst.com/)
Fiji/ImageJ (https://imagej.net/software/fiji/downloads)
Prism 9 (https://www.graphpad.com/features)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Kunduri, G. and Acharya, J. K. (2024). Live Imaging and Analysis of Meiotic Cytokinesis in Drosophila Testes. Bio-protocol 14(2): e4918. DOI: 10.21769/BioProtoc.4918.
分类
发育生物学 > 细胞生长和命运决定
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link