- EN - English
- CN - 中文
Generation of Mature Toxoplasma gondii Bradyzoites in Human Immortalized Myogenic KD3 Cells
在人类永生化肌原 KD3 细胞中生成成熟的刚地弓形虫裂殖体
发布: 2024年01月05日第14卷第1期 DOI: 10.21769/BioProtoc.4916 浏览次数: 2005
评审: Marcelo S. da SilvaAnonymous reviewer(s)
Abstract
Toxoplasma gondii is a zoonotic protozoan parasite and one of the most successful foodborne pathogens. Upon infection and dissemination, the parasites convert into the persisting, chronic form called bradyzoites, which reside within cysts in muscle and brain tissue. Despite their importance, bradyzoites remain difficult to investigate directly, owing to limited in vitro models. In addition, the need for new drugs targeting the chronic stage, which is underlined by the lack of eradicating treatment options, remains difficult to address since in vitro access to drug-tolerant bradyzoites remains limited. We recently published the use of a human myotube-based bradyzoite cell culture system and demonstrated its applicability to investigate the biology of T. gondii bradyzoites. Encysted parasites can be functionally matured during long-term cultivation in these immortalized cells and possess many in vivo–like features, including pepsin resistance, oral infectivity, and antifolate resistance. In addition, the system is scalable, enabling experimental approaches that rely on large numbers, such as metabolomics. In short, we detail the cultivation of terminally differentiated human myotubes and their subsequent infection with tachyzoites, which then mature to encysted bradyzoites within four weeks at ambient CO2 levels. We also discuss critical aspects of the procedure and suggest improvements.
Key features
• This protocol describes a scalable human myotube-based in vitro system capable of generating encysted bradyzoites featuring in vivo hallmarks.
• Bradyzoite differentiation is facilitated through CO2 depletion but without additional artificial stress factors like alkaline pH.
• Functional maturation occurs over four weeks.
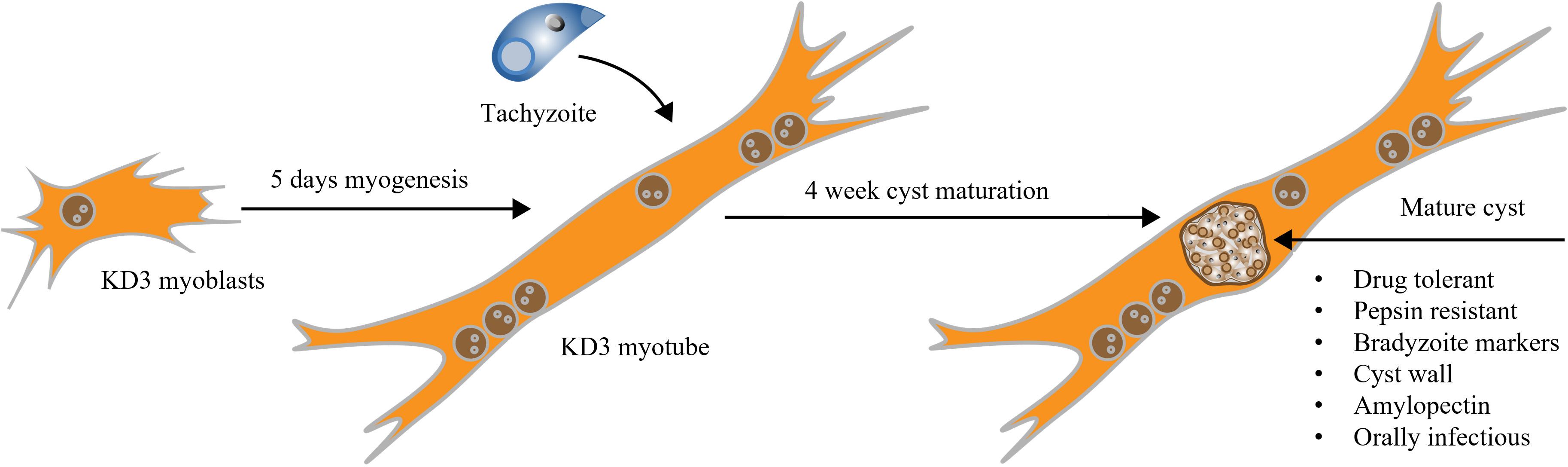
Graphical overview
Background
The obligate intracellular parasite Toxoplasma gondii infects nearly all warm-blooded animals and an estimated quarter of the human global population (Molan et al., 2019). The World Health Organization ranked T. gondii to be an important and common food-borne protozoan, with one of the highest disease burdens worldwide (Torgerson et al., 2015). It commonly manifests as an opportunistic pathogen in AIDS patients and other immunocompromised individuals (Luft and Remington, 1992). In contrast, most healthy individuals can effectively control the acute infection through their immune system (Denkers and Gazzinelli, 1998). Semi-dormant bradyzoites can persist for the duration of the host’s life in cysts within mainly the brain or muscle tissue, being resistant to current treatment options (Dubey, et al., 1998; Watts et al., 2015; Barrett et al., 2019). Bradyzoites can reactivate if the immune pressure abates (e.g., via AIDS, transplantation, etc.) leading to the emergence of severe clinical manifestations. Tissue cysts are a major source of transmission via the consumption of undercooked meat from infected animals (Gabriël et al., 2022). Hence, the investigation of bradyzoite biology including chemotherapy development is much needed.
In the past, corresponding research efforts were hindered by the lack of an appropriate in vitro model. Either isolated cysts from mice brain homogenates or functionally immature bradyzoites derived from fibroblast cell cultures were used. Many traits of bradyzoites, such as drug-tolerance mechanisms, remain challenging to study in vitro. Spontaneous conversion of tachyzoites to bradyzoites has been characterized in murine C2C12 myotubes (Guimarães et al., 2008; Ferreira-da-Silva et al., 2009; Swierzy and Lüder, 2015). We recently showed that immortalized human myoblasts (called KD3) are also suitable for long-term bradyzoite culture (Christiansen et al., 2022). KD3 myoblasts harbor transgenes for mutated cyclin-dependent kinase 4, cyclin D1, and a human telomerase reverse transcriptase (hTERT), resulting in the immortalization of these primary subcutaneous muscle cells (Shiomi et al., 2011). In differentiated KD3 myotubes, cystogenic strains of T. gondii can be matured over a period of four weeks, during which the cysts develop structural and functional traits of in vivo cysts (Christiansen et al., 2022) and express bradyzoite and cyst markers, such as BAG1, CC2, glycans, a discernible cyst wall, as well as amylopectin granules. In addition, the cysts become tolerant to the antifolates pyrimethamine and sulfadiazine and are orally infectious to mice. They gradually develop resistance to pepsin digestion and survive storage at 4 °C and exposure to 55 °C (Christiansen et al., 2022).
With this protocol, we aim to facilitate the use of in vitro T. gondii cysts in studies to reduce animal experiments and to increase the exploration of this comparatively understudied form of T. gondii.
Materials and reagents
Cell lines
BJ-5ta human foreskin fibroblast (HFF) cells (ATCC CRL-4001)
Toxoplasma gondii Prugniaud- tdTomato (Chtanova et al., 2008) or any other cystogenic strain
KD3 myoblasts (Shiomi et al., 2011) or other immortalized human myogenic cell lines with similar properties
Note: We know of four human immortalized myogenic cell lines (and derivatives thereof) described in the literature (Zhu et al., 2007; Shiomi et al., 2011; Thorley et al., 2016; Massenet et al., 2020). KD3 cells used here were initially obtained from N. Hashimoto. Inquiries for KD3 cells should now be addressed to Dr. Tohru Hosoyama, Dept. of Musculoskeletal Disease, The Geroscience Research Center, National Center for Geriatrics and Gerontology, 7-430 Morioka-cho, Obu City, Aichi Prefecture, Japan (toruhoso@ncgg.go.jp). Upon completion of an MTA, the cells might be obtained in Europe from the authors, or in the US from Dr. L. Weiss, Albert Einstein College of Medicine, 1300 Morris Park Avenue, Forchheimer Building, Bronx, NY 10461, USA (louis.weiss@einsteinmed.edu). LHCN-M2 cells (Zhu et al., 2007) are commercially available from Evercyte. Cells from Massenet et al. might be obtained from the respective authors.
Reagents
Dulbecco’s modified Eagle’s medium (DMEM) (Gibco Life Technologies, catalog number: 12800-082)
Glucose (Carl-Roth, catalog number: X997.2)
L-glutamine (Thermo Fisher Scientific, catalog number: 25030081)
Sodium pyruvate (Capricorn Scientific, catalog number: NPY-B)
Penicillin/streptomycin (Capricorn Scientific, catalog number: PS-B)
Bovine serum, heat inactivated, iron fortified (Capricorn Scientific, catalog number: CS-IF-1A)
Fetal bovine serum, heat inactivated (Capricorn Scientific, catalog number: FBS-12A)
Fetal bovine serum, heat inactivated low endotoxin (Capricorn Scientific, catalog number: FBS-LE-12A)
Ultroser® G (Cytogen, catalog number: 15950-017)
Donor horse serum (Capricorn Scientific, catalog number: DHS-1A)
Human recombinant insulin (PAN-Biotech, catalog number: P-2701001)
Insulin Lispro (Sanofi, catalog number: 12910612)
Human holo-transferrin (Sigma, catalog number: TO665)
Na2SeO3 (Sigma-Aldrich, catalog number: 214485)
RPMI 1640 pH 7.4 (containing 4 mM L-glutamine) (Sigma-Aldrich, catalog number: R1383)
HEPES (Sigma-Aldrich, catalog number: H3375)
DMSO (AppliChem, catalog number: A3672)
Collagen I from rat tail (Corning, catalog number: 354236)
NaCl (Merck catalog number: 1.06404)
KCl (Merck, catalog number: 1.04936)
KH2PO4 (Merck, catalog number: 1.04877)
Na2HPO4·2H2O (Merck, catalog number: 1.06580)
Acetic acid (Carl Roth, catalog number: 3738.4)
0.05% trypsin/0.53 mM EDTA (Capricorn Scientific, catalog number: TRY-1B10)
Media and solutions
70% ethanol for sterilization
HFF growth medium (see Recipes)
Tachyzoite medium (see Recipes)
Myoblast growth medium (see Recipes)
Myoblast differentiation medium (see Recipes)
Cyst medium (see Recipes)
Freezing medium (see Recipes)
Rat tail collagen type I (see Recipes)
1× Trypsin-EDTA solution in PBS (w/o Ca2+ and Mg2+)
Phosphate-buffered saline (PBS) (w/o Ca2+and Mg2+)
Note: All media have to be sterile.
Material
T-25 or T-75 cell culture flasks with filter caps (TPP, catalog number: 90026 or 90076)
Microtiter plates 6 well or 96 well (TPP, catalog number: 92006 or 92048)
15 mL and 50 mL conical centrifuge tubes (TPP, catalog number: 91015 and 91050)
1.5 mL cryovials (Nalgene, catalog number: 5000-1020)
Pipette tips [10, 200, 1,000 µL (Various brands)]
Sterile syringe filters (0.22 μm) (TPP, catalog number: 99722)
C-Chip disposable hemocytometer (NanoEnTek Inc., DHC-N01) or Neubauer hemocytometer
Syringes (20 mL) (Luer Lock, Injekt, catalog number: 4606736V)
Blunt cannula (27 G) (Sterican, catalog number: 9180117)
Note: Examples for providers/manufacturers are given; similar material from others will probably work as well.
Recipes
HFF growth medium
DMEM pH 7.2 (containing 3.75 g/L NaHCO3, 25 mM glucose, 4 mM L-glutamine, and 1 mM sodium pyruvate), supplemented with 1× penicillin/streptomycin and 10% bovine serum. Store at 4 °C.
Tachyzoite medium
HFF growth medium but with only 1% fetal bovine serum. Store at 4 °C.
Myoblast growth medium
DMEM pH 7.2 (containing 3.75 g/L NaHCO3, 25 mM glucose, 4 mM L-glutamine, and 1 mM sodium pyruvate), supplemented with 1× penicillin/streptomycin, 20% fetal bovine serum, and 2% Ultroser® G. Store at 4 °C.
Myoblast differentiation medium
DMEM pH 7.2 (containing 3.75 g/L NaHCO3, 25 mM glucose, 4 mM L-glutamine, and 1 mM sodium pyruvate), supplemented with 1× penicillin/streptomycin, 2% donor horse serum, 10 µg/mL human recombinant insulin (alternatively, Insulin Lispro), 5 µg/mL human holo-transferrin, and 10 nM Na2SeO3. Store at 4 °C.
Note: Lower concentrations of insulin might also work, according to literature.
Cyst medium
RPMI 1640 pH 7.4 (containing 4 mM L-glutamine), supplemented with 5 mM glucose, 1× penicillin/streptomycin, 50 mM HEPES, 2% donor horse serum, 10 µg/mL human recombinant insulin (alternatively, Insulin Lispro), 5 µg/mL human holo-transferrin, and 10 nM Na2SeO3. Store at 4 °C.
Freezing medium
90% heat-inactivated fetal bovine serum low endotoxin and 10% DMSO. Store at 4 °C.
Rat tail collagen
Collagen I from rat tail dissolved at a stock concentration of 0.1 mg/mL in 100 mM acetic acid. Use 1:6.67 in water. Store at 4 °C.
1× Trypsin-EDTA solution
0.05% trypsin/0.53 mM EDTA in PBS (w/o Ca2+ and Mg2+)
Phosphate-buffered saline (PBS) without calcium and magnesium
MilliQ water supplemented with 137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, and 6.5 mM Na2HPO4·2H2O at pH 7.4.
Equipment
Humidified CO2 cell incubator at 36.7 °C, 10% CO2 (Thermo Fisher Scientific, model: Heracell 150i CO2 incubator)
Incubator at 36.7 °C, ambient CO2 (Binder Inkubator, model: KB 240)
Safety cabinet for BSL-2 work (Luft- und Reinraumtechnik GmbH, model: BDK S1200 laminar flow workbench)
Water bath at 37 °C (Memmert GmbH & Co. KG, model: WNB 14)
Adjustable micropipettes 2–20 µL, 20–200 µL, 100–1,000 µL (Eppendorf GmbH, model: Research Plus micropipette)
Pipetting aid for serological pipettes (Brand GmbH, model: Accu-Jet Pro pipette)
Vacuum aspirator system, Integra Biosciences Vacusafe Comfort Aspiration system (Integra, model: 10590273)
Inverted light microscope with phase contrast, differential interference contrast, or similar optics (Nikon, model: Eclipse Ts2 inverted microscope)
Centrifuge with swing-out rotor and refrigeration (Hettich GmbH & Co. KG, model: Rotina 380R centrifuge 1706)
CoolCell LX Cell Freezing Vial Containers (Fisher Scientific, Corning, catalog number: 15572771) or similar cryo-freezing container
Refrigerator 4 °C (AEG, model: RTB415E2AW)
-20 °C freezer (Liebherr, model: Mediline LGex 3410)
-70 °C freezer (SANYO, model: VIP Series MDF-U53V ultra-low temperature freezer)
Liquid nitrogen storage capabilities
Note: Examples for providers/manufacturers are given; similar material from others will probably work as well.
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Maus, D., Curtis, B., Warschkau, D., Betancourt, E. D., Seeber, F. and Blume, M. (2024). Generation of Mature Toxoplasma gondii Bradyzoites in Human Immortalized Myogenic KD3 Cells. Bio-protocol 14(1): e4916. DOI: 10.21769/BioProtoc.4916.
分类
微生物学 > 微生物生理学
生物科学 > 生物技术 > 微生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link