- EN - English
- CN - 中文
In vitro Assay to Examine the Function of Tears on Corneal Epithelial Cells
体外检测泪液对角膜上皮细胞功能的影响
发布: 2024年01月05日第14卷第1期 DOI: 10.21769/BioProtoc.4910 浏览次数: 2685
评审: Alberto RissoneAbhishek vatsAnonymous reviewer(s)
Abstract
Tears contain numerous secreted factors, enzymes, and proteins that help in maintaining the homeostatic condition of the eye and also protect it from the external environment. However, alterations to these enzymes and/or proteins during pathologies such as mechanical injury and viral or fungal infections can disrupt the normal ocular homeostasis, further contributing to disease development. Several tear film components have a significant role in curbing disease progression and promoting corneal regeneration. Additionally, several factors related to disease progression are secreted into the tear film, thereby serving as a valuable reservoir of biomarkers. Tears are readily available and can be collected via non-invasive techniques or simply from contact lenses. Tears can thus serve as a valuable and easy source for studying disease-specific biomarkers. Significant advancements have been made in recent years in the field of tear film proteomics, lipidomics, and transcriptomics to allow a better understanding of how tears can be utilized to gain insight into the etiology of diseases. These advancements have enabled us to study the pathophysiology of various disease states using tear samples. However, the mechanisms by which tears help to maintain corneal homeostasis and how they are able to form the first line of defense against pathogens remain poorly understood and warrant detailed in vitro studies. Herein, we have developed an in vitro assay to characterize the functional importance of patient isolated tears and their components on corneal epithelial cells. This novel approach closely mimics real physiological conditions and could help the researchers gain insight into the underlying mechanisms of ocular pathologies and develop new treatments.
Key features
• This method provides a new technique for analyzing the effect of tear components on human corneal epithelial cells.
• The components of the tears that are altered in response to diseases can be used as a biomarker for detecting ocular complications.
• This procedure can be further employed as an in vitro model for assessing the efficacy of drugs and discover potential therapeutic interventions.
Keywords: Ocular diseases (眼部疾病)Background
Tears are a vital component of the ocular surface, serving as a first line of defense against pathogens and extreme changes in the environment [1, 2] One of the main functions of tears is to act as lubricant, keeping the eye moist while also protecting it from physical damage [2]. Tears also help to maintain conjunctival and corneal health by providing nutrition, regulating optical properties, and boosting tissue cooling. The unique composition of tears also protects the ocular surface against various bacterial, fungal, and viral infections. The tear film is comprised of three layers: the innermost mucous layer, the middle aqueous part, and the outermost lipid layer. The biological composition of metabolites, electrolytes, mucins, lipids, water, proteins, salts, and organic molecules that are present in tears can be used to distinguish healthy and diseased states [1]. Several studies have shown how tears can be used as an indicator of ocular health and pathology, for example in diabetic retinopathy [3], dry eye disease [4], glaucoma, Graves’ ophthalmopathy, and in some ocular tumors [5, 6].
Given that tears are readily available and can be collected via non-invasive procedures, they have recently been recognized as more valuable tools in the identification of ocular health and in the diagnosis of diseased states when compared to other biological fluids. Therefore, studies are being dedicated towards characterizing the composition of tears and understanding how changes in this composition relate to pathology. In several ocular disorders, tears have been proposed to promote inflammation, and the precise secretome of the tears directly relates to the ocular microenvironment [6]. However, characterizing how tears affect ocular health and more specifically how tears affect corneal epithelial cells remains poorly understood. Herein, we describe procedures that can be easily implemented to study how healthy vs. diseased tears affect the corneal epithelial cells in vitro. This protocol is based on our recently published paper, which focuses on how the tear proteins of Persistent Epithelial Defect (PED) patients have the potential to alter the inflammatory, fibrotic, and corneal epithelial barrier profile of other healthy cornea cells [7]. Taken together, we can infer that patient-derived tears can be a valuable source of factors to create a disease-specific in vitro model to study the key features of the pathology. This system can also be used to study the patient-specific clinicopathological features.
There are standard protocols describing the methods for collecting tears, extracting the protein, and using it for biochemical characterization, proteomic analysis, and inflammatory cytokines analysis. Recently, tears have been explored as a non-invasive method to identify disease-specific markers. Recent reports highlight the presence of exosomes in keratoconus tears, hypothesized as a communication vehicle [8]. The in vitro model used for studying disease pathology, such as in dry eye, allergy, or inflammatory models, is based on using a single growth factor/cytokine, which does not represent the actual pathology, as the disease state is governed by multiple factors such as cytokines, growth factors, and metabolites. The novel aspect of the described protocol is the use of patient-derived factors in the form of tears, which is the true representation of the diseased state (including growth factors, cytokines, and metabolites). Co-culturing patient-specific and control tears with relevant cells (corneal epithelial cells in this case) will provide an in vitro model that truly depicts the diseased state, whereas the healthy individual will be taken as a control. To the best of our knowledge, the ease of collecting tears and using it as a supplement in cell culture has never been explored previously, and this is the first study describing the detailed methodology for establishing a tear-based in vitro model of ocular diseases.
This protocol highlights the effect of patient-derived tears on corneal epithelial cells’ morphology and gene expression pattern. The procedure to collect tears can be done using either the Schirmer strip or the plastic capillary tube method. Sampling with Schirmer strip is relatively easy, rapid, reliable, and free of risk, whereas obtaining tears from capillary tube requires a degree of practice and experience; this procedure is also interrupted by blinking and the investigator needs to hold the tube for the duration of the sampling process. Hence, the protocol developed using the Schirmer’s test strip is easy to carry out, reproducible, and can be used to further understand ocular disease progression. Proteins are isolated post tear collection using the Schirmer’s test strip from both healthy and diseased individuals. Isolated proteins are then used to treat human corneal limbal epithelial (HCLE) cells at a specified concentration for a defined period (48 h in our study) until visual changes are observed. Next, morphological changes in inflammatory or fibrotic gene expression are recorded and studied. Matrix metalloproteinases (MMPs) are iron- and calcium-dependent enzymes that degrade the corneal collagen and the extracellular matrix. The level of MMP9 activity in the tear fluids has been regarded as an indicator of ocular inflammation and damage in dry eye disease, vernal keratoconjunctivitis, and glaucoma. Thus, MMP9 activity of the tears of PED patients has been studied via gelatin zymography and is represented in Figure 1. This novel assay uses tear fluid as a medium for testing and analyzing biomarkers, allowing for a more cost-effective and non-invasive diagnosis. The technique is also beneficial for tracking disease progression, as it can be used to detect changes in biomarkers over time. Furthermore, this method presents a convenient and time-saving method for healthcare professionals to monitor the health of their patients. Tears can be used for in vitro drug testing as a more effective and efficient means to test for the efficacy and safety of drugs. This model also mimics the in vivo conditions and is thus useful in drug testing.
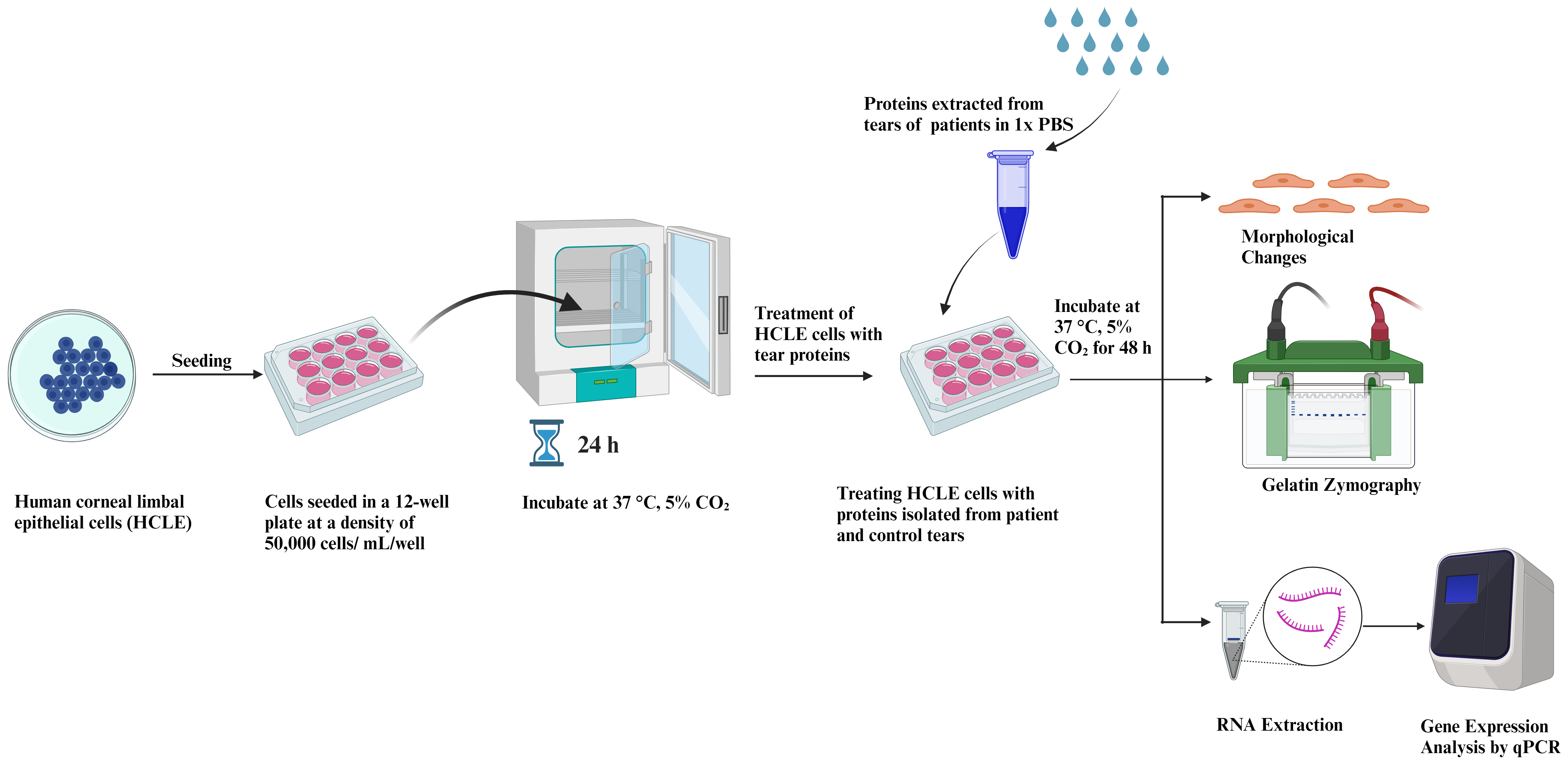
Figure 1. Graphical representation of human corneal limbal epithelial (HCLE) cells treated with tear protein extracts and their downstream processing
Materials and reagents
50 mL conical centrifuge tubes (Thermo Fisher Scientific, catalog number: 339652)
15 mL conical centrifuge tubes (Thermo Fisher Scientific, catalog number: 339650)
100 mm culture dish (tissue culture treated) (Corning, catalog number: 430167)
12-well plate (tissue culture treated) (Thermo Fisher Scientific, catalog number: 173095)
10 mL serological pipettes (Thermo Fisher Scientific, catalog number: 170356N)
1,000 μL micro tip (Thermo Fisher Scientific, catalog number: 90030210-P)
200 μL micro tip (Thermo Fisher Scientific, catalog number: 90030130-P)
10 μL micro tip (Thermo Fisher Scientific, catalog number: 90030000-P)
1.5 mL microcentrifuge tube (Tarsons, catalog number: 500010)
Human corneal limbal epithelial (HCLE) cells (a kind gift from Dr. Ilene Gipson, Harvard Medical School, Schepen Eye Research Institute of Mass Eye and Ear)
Keratinocyte-serum free media (KSFM), supplemented with bovine pituitary extract and epidermal growth factor (EGF) (Gibco, Thermo Fisher Scientific, catalog number: 10724-011-500ML)
Penicillin/streptomycin solution (100×) (Diagnovum, catalog number: D910-100ML)
TrypLETM Express w/o phenol red (Gibco, Thermo Fisher Scientific, catalog number: 12604-013-100ML)
Minimal essential medium (MEM) (Gibco, Thermo Fisher Scientific, catalog number: 32571-036-500ML)
Fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific, catalog number: 10270-106-500ML)
Dulbecco’s PBS w/o Ca2+, Mg2+ and phenol red (1×) (Diagnovum, catalog number: D402-500ML)
Trypan blue (Thermo Fisher Scientific, catalog number: T10282)
Ammonium persulphate (APS) (Invitrogen, Thermo Fisher Scientific, catalog number: HC2005)
SureCastTM TEMED (Invitrogen, Thermo Fisher Scientific, catalog number: HC2006)
SureCastTM 40 % (w/v) acrylamide (29:1 acrylamide:bis-acrylamide) (Invitrogen, Thermo Fisher Scientific, catalog number: HC2040)
2.5× Tris-SDS buffer (pH 8.8) (HIMEDIA, catalog number: MB039-500ML)
5× Tris-SDS buffer (pH 6.8) (HIMEDIA, catalog number: MB040-500ML)
Gelatin, Hi-LRTM (HIMEDIA, catalog number: GRM019-500G)
0.2 μm Polyethersulfone (PES) syringe filter (mdi Membrane Technologies, catalog number: SYKG0601MNXX204)
Mini-RNA Isolation kit (Qiagen, catalog number: 74004)
Verso c-DNA Synthesis kit (Thermo Fisher Scientific, catalog number: AB1453)
SYBR Green (Applied Biosystems, Thermo Fisher Scientific, catalog number: A25742)
NuPage LDS sample buffer (4×) (Invitrogen, Thermo Fisher Scientific, catalog number: 2463558)
Calcium chloride (CaCl2) (HIMEDIA, catalog number: GRM3906-500G)
Triton X-100 (HIMEDIA, catalog number: MB031-100M)
Coomassie brilliant blue R-250 (HIMEDIA, catalog number: MB153-25G)
Methanol (Rankem, catalog number: M0140)
Acetic acid (Rankem, catalog number: A0060)
Sodium chloride (HIMEDIA, catalog number: MB023-500G)
1 mL syringes (DISPOVAN, model: U-40 Insulin syringe)
Schirmer tear test strips/tear touch (Madhu Instruments Pvt. Ltd.)
Gloves (Kimberly-Clark, catalog number: KM50601)
Kimwipes (Kimtech Science, catalog number: 34120)
Complete KSFM+GF (500 mL) (see Recipes)
Basal media: incomplete KSFM-GF (see Recipes)
1% Triton X-100 solution (see Recipes)
Developing buffer (see Recipes)
Coomassie staining solution (see Recipes)
Destaining solution (see Recipes)
Recipes
Complete KSFM+GF (500 mL)
Bovine pituitary extract, 25 μg/mL (half vial provided)
EGF (0.2 ng/mL)
CaCl2 (0.4 mM)
Penicillin/streptomycin 100× antibiotic (5 mL to achieve a final concentration of 1×)
Basal media: incomplete KSFM-GF
Penicillin/streptomycin 100× antibiotic (5 mL to achieve a final concentration of 1×)
1% Triton X-100 solution
1% Triton X-100 (v/v)
Autoclaved Milli-q water
Developing buffer
10 mM Tris buffer
200 mM NaCl
6 mM CaCl2
pH 7.4
Coomassie staining solution
Coomassie brilliant blue R-250 (0.05%)
Methanol (50% v/v)
Acetic acid (10% v/v)
Make up the remaining volume with Milli-Q water
Destaining solution
Acetic acid (10% v/v)
Methanol (50% v/v)
Milli-Q water (40% v/v)
Equipment
Swinging bucket centrifuge (NEUATION, model: ifuge UC02; catalog number: UC- 2002- EU/UK/AUS)
Tabletop centrifuge (Thermo Fisher Scientific, model: Sorvall Legend Micro 21R; catalog number: 75002447)
Class II biological safety cabinet (ESCO, model: Class II BSC)
Cell culture CO2 incubator (ESCO, model: CCL-170B-8-UV)
EVOSTM XL Core imaging system (Thermo Fisher Scientific, model: AMEX1200)
Automated cell counter (Thermo Fisher Scientific, model: CountlessTM 3 AMQAX2000)
Hemacytometer (Thermo Fisher Scientific, model: CountlessTM Reusable Slide, catalog number: A25750)
NanoDropTM Lite spectrophotometer (Thermo Fisher Scientific, catalog number: ND-LITE)
Gel Doc (Azure Biosystems, model: c600)
Real-time PCR (Azure Biosystems, model: Ceilo 6)
Thermocycler (Bio-Rad, model: T1000)
Rocker shaker (Scientek Hub, model: SR-1)
SureCastTM glass plates for PAGE ((Invitrogen, Thermo Fisher Scientific, catalog number: HC1001)
Mini gel tank (Invitrogen, Thermo Fisher Scientific, AMEX 1200; catalog number: A25977)
-80°C laboratory freezer (Thermo Fisher Scientific, model: Forma 900)
Milli-Q (Elga, model: PQ00018907)
Vortex (NEUATION, model: iSWIX)
Pipettes (1,000, 200, 10, 2.5 μL) (Eppendorf, model: Research Plus)
Software and datasets
Prism 9 (GraphPad Prism version 9.4.1)
BioRender for graphical images (https://www.biorender.com/)
Azure Cielo Manager software (version 1.0.4.0)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Mondal, M., Vohra, M., Sangwan, J., Verma, S., Coulson-Thomas, V. J., Chandru, A., Bhowmick, T., Sangwan, V. S., Acharya, M. and Tiwari, A. (2024). In vitro Assay to Examine the Function of Tears on Corneal Epithelial Cells. Bio-protocol 14(1): e4910. DOI: 10.21769/BioProtoc.4910.
分类
生物化学 > 蛋白质 > 分离和纯化
细胞生物学 > 基于细胞的分析方法 > 炎症反应
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













