- EN - English
- CN - 中文
Dissociation and Culture of Adult Mouse Satellite Glial Cells
成年小鼠卫星胶质细胞的分离和培养
发布: 2023年12月20日第13卷第24期 DOI: 10.21769/BioProtoc.4906 浏览次数: 2385
评审: Olga KopachChristian VaegterAnonymous reviewer(s)
Abstract
Satellite glial cells (SGCs) are a type of glial cell population that originates from neural crest cells. They ultimately migrate to surround the cell bodies of neurons in the ganglia of the peripheral nervous system. Under physiological conditions, SGCs perform homeostatic functions by modifying the microenvironment around nearby neurons and provide nutrients, structure, and protection. In recent years, they have gained considerable attention due to their involvement in peripheral nerve regeneration and pain. Although methods for culturing neonatal or rat SGCs have long existed, a well-characterized method for dissociating and culturing adult SGCs from mouse tissues has been lacking until recently. This has impeded further studies of their function and the testing of new therapeutics. This protocol provides a detailed description of how to obtain primary cultures of adult SGCs from mouse dorsal root ganglia in approximately two weeks with over 90% cell purity. We also demonstrate cell purity of these cultures using quantitative real-time RT-PCR and their functional integrity using calcium imaging.
Key features
• Detailed and simplified protocol to dissociate and culture primary satellite glial cells (SGCs) from adult mice.
• Cells are dissociated in approximately 2–3 h and cultured for approximately two weeks.
• These SGC cultures allow both molecular and functional studies.
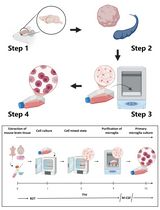
Graphical overview
Dissociation and culture of mouse satellite glial cells
Background
Satellite glial cells (SGCs) are a major cellular component of the peripheral nervous system, surrounding the cell bodies of sensory neurons in dorsal root (DRG) and trigeminal ganglia (Hanani, 2005). Long thought to be merely supportive of neurons, increasing evidence suggests that SGCs are dynamic regulators of neuronal processing, including pain on the peripheral nervous system (Ji et al., 2013). Pain affects more than 1.5 billion people worldwide, with hundreds of millions suffering from unrelieved chronic pain (Kennedy et al., 2014). Given the overuse of opioid prescriptions to treat chronic pain, new therapeutics are urgently needed (Grosser et al., 2017).
While there is a growing consensus that SGCs play a role in important physiological and pathological pain mechanisms (Hanani and Spray, 2020; Gazerani, 2021; Andreeva et al., 2022) and may provide new therapeutic targets for pain relief, our knowledge of SGC biology is still limited because of the lack of techniques to study these cells in vitro. Previous protocols for the dissociation and culture of SGCs have been generated from neonatal and immature tissues, contained neuronal and non-neuronal cells, or mostly used rats (Chen et al., 2008; Belzer et al., 2010; de Corato et al., 2011; Poulsen et al., 2014; Wang et al., 2019). Recently, there have been publications using cultured SGCs from adult mice (Su et al., 2022; Tonello et al., 2023). However, a detailed and well-characterized protocol for the dissociation and culture of mouse SGCs is still lacking.
Here, we describe a step-by-step protocol used in our recent publication (Tonello et al., 2023) that dissociates adult SGCs from mouse DRG tissues. The protocol results in cultures that are more than 90% pure and take approximately 10–14 days to be suitable for molecular and functional testing. As a proof of concept, we have also characterized these cultures for the transcriptional expression of SGC-enriched genes, such as the endothelin receptor type B (EDNRB), and functional responses to a specific EDNRB agonist by calcium imaging.
Materials and reagents
Biological materials
CD1 mouse (6–12 weeks) (Charles River, catalog number: 022)
Reagents
Hanks’ balanced salt solution (HBSS) without Ca2+/Mg2+ (Thermo Fisher Scientific, catalog number: 14175095)
1 M HEPES (Thermo Fisher Scientific, catalog number: 15630080)
Penicillin-Streptomycin (P/S), 10,000 U/mL (Thermo Fisher Scientific, catalog number: 15140122)
Papain (buffered aqueous suspension, ≥16 units/mg protein) (Millipore Sigma, catalog number: P3125)
Collagenase from Clostridium histolyticum (Millipore Sigma, catalog number: C6885)
Dulbecco’s modified Eagle medium (DMEM), low glucose (Thermo Fisher Scientific, catalog number: 11885084)
Fetal bovine serum (FBS), heat inactivated (Fisher Scientific, catalog number: MT35011CV)
Amphotericin B (Thermo Fisher Scientific, catalog number: 15290026)
Solutions
Note: Prepare all solutions in a laminar flow cell culture hood.
HBSS dissociation solution (see Recipes)
Papain solution (see Recipes)
Collagenase stock (see Recipes)
Collagenase solution (see Recipes)
DMEM culture medium (see Recipes)
Recipes
HBSS dissociation solution (store at 4 °C for up to six months)
Reagent Final concentration Quantity HBSS n/a 490 mL HEPES 1% (v/v) 5 mL P/S 1% (v/v) 5 mL Total n/a 500 mL Papain solution (freshly prepared and used)
Reagent Final concentration Quantity Papain 40 units total 108 μL HBSS dissociation solution n/a 3 mL Total n/a 3.108 mL Collagenase stock (aliquot at 200 μL, and store at -20 °C for up to six months)
Reagent Final concentration Quantity Collagenase 22.5 mg/mL 100 mg HEPES 1% (v/v) 44 μL HBSS n/a 4.4 mL Total n/a 4.444 mL Collagenase solution (freshly prepared and used)
Reagent Final concentration Quantity Collagenase stock 1.5 mg/mL 200 μL HBSS dissociation solution n/a 2.8 mL Total n/a 3.0 mL DMEM culture medium (store at 4 °C for up to six months)
Reagent Final concentration Quantity FBS 10% (v/v) 50 mL P/S 1% (v/v) 5 mL Amphotericin B 1% (v/v) 5 mL DMEM, low glucose n/a 440 mL Total n/a 500 mL
Laboratory supplies
Isoflurane (Henry Schein, catalog number: 1182097)
Spray bottle with 70% ethanol (Fisher Scientific, catalog number: BP82031GAL)
Tissue culture dish (35 × 10 mm) (Fisher Scientific, catalog number: 08-772A)
Microtubes, 1.7 mL (Fisher Scientific, catalog number: 14-222-168)
Centrifugation tubes, 50 mL (Fisher Scientific, catalog number: 06-443-18)
Cell strainer 40 μm (Fisher Scientific, catalog number: 22-363-547)
Cell strainer 10 μm (PluriSelect, catalog number: 43-50010-03)
Cell culture 12-well microplates (Fisher Scientific, catalog number: 07-000-202)
Cover glass (Fisher Scientific, catalog number: 22-050-232)
Equipment
Stereomicroscope system (Olympus, catalog number: SZ51)
Biosafety cabinet (Fisher Scientific, catalog number: NC0986267)
Isotemp water bath (Fisher Scientific, catalog number: FSGPD2S)
Water-jacketed CO2 incubator (Fisher Scientific, catalog number: 13-998-078)
Sorvall Legend Micro 21R microcentrifuge (Thermo Fisher Scientific, catalog number: 75002445)
Centrifuge 5702 (Eppendorf, catalog number: 022628102)
Standard scissors (Fine Science Tools, catalog number: 14002-12)
Forceps (World Precision Instruments, catalog number: 501987)
Student spring scissors (Fine Science Tools, catalog number: 91500-09)
Dumont #5 forceps (Fine Science Tools, catalog number: 11251-20)
Gilson (or equivalent) pipettes and tips (P20/P200/P1000) (Fisher Scientific, catalog number: various)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Tonello, R., Davidson, S. and Berta, T. (2023). Dissociation and Culture of Adult Mouse Satellite Glial Cells. Bio-protocol 13(24): e4906. DOI: 10.21769/BioProtoc.4906.
分类
神经科学 > 细胞机理 > 细胞分离和培养
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link