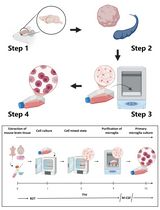
- EN - English
- CN - 中文
Streamlined Methods for Processing and Cryopreservation of Cell Therapy Products Using Automated Systems
使用自动化系统处理和冷冻保存细胞治疗产品的简化方法
(*contributed equally to this work) 发布: 2023年12月20日第13卷第24期 DOI: 10.21769/BioProtoc.4900 浏览次数: 3137
评审: Jan HuebingerKrishna NakuluriAnonymous reviewer(s)
Abstract
Streamlined procedures for processing and cryopreservation of cell therapies using good laboratory practices are integral to biomanufacturing process development and clinical applications. The protocol herein begins with the preparation of human cell types cultured as adherent (i.e., mesenchymal stromal cells, MSCs) or suspension cells (i.e., peripheral blood mononuclear cells, PBMCs) to comprehensively demonstrate procedures that are applicable to commonly used primary cell cultures. Cell processing steps consist of preparing high yields of cells for cryopreservation using instruments routinely used in cell manufacturing, including the Finia® Fill and Finish System and a controlled-rate freezer. The final steps comprise the storage of cells at subzero temperatures in liquid nitrogen vapor phase followed by the analysis of cell phenotypes before and after processing and cryopreservation, along with cell quality metrics for validation. Additionally, the protocol includes important considerations for the implementation of quality control measures for equipment operation and cell handling, as well as Good Laboratory Practices for cell manufacturing, which are essential for the translational use of cell therapies.
Key features
• The protocol applies to small- or large-scale manufacturing of cell therapy products.
• It includes streamlined procedures for processing and cryopreservation of cells cultured as adherent cells (MSCs) and suspension cells (PBMCs).
• Provides temperature control and rapid partitioning of sample in cryopreservation solution to maintain high viability of a range of cell types throughout the procedures.
• This protocol employs the Finia® Fill and Finish System and a controlled-rate freezer.
Graphical overview
Background
Cell therapy investigations rely on ex vivo operations to adequately preserve the viability and functionality of the cell products prior to in vivo administration (Li et al., 2019). Process development for cell products requires standard operations with controlled systems, which should be monitored throughout the process of end-to-end manufacturing. Clinical laboratories routinely cryopreserve cells using cryopreservation solutions and operating conditions that need to be tightly controlled and amenable to the cell type for optimal cell viability and yield. The use of automated equipment that combines multiple manufacturing processes, such as fill-finish systems and controlled-rate freezers, can offer several advantages over manual operations including automation, better control of processing, and efficiency, thereby avoiding potential compromises to the viability and yield of cell products (van der Walle et al., 2021). Together, these systems can be employed and programmed to optimize the operations for cell cryopreservation at end-stage manufacturing as well as starting materials upstream of manufacturing.
Multiple cell types have been evaluated as cell therapies for clinical use to treat a broad spectrum of clinical indications. Depending on the cell type, specific reagents and operating conditions need to be considered for ex vivo manufacturing expansions to generate high yields for therapeutic dosages (Galipeau and Sensébé, 2018; Pigeau et al., 2018). For example, anchorage-dependent cells require adherence to substrates (e.g., plastic cultureware, microcarriers) to proliferate, whereas other cells proliferate in suspension without substrates (Merten, 2015; Cuesta-Gomez et al., 2023). Typically, cell therapy products are initially isolated from tissues or collected by apheresis from individuals and subsequently undergo various process development procedures to isolate, expand (i.e., proliferate), and harvest the cells (Bunnell et al., 2008; Palen et al., 2021). Herein, procedures that are applicable for both adherent (i.e., mesenchymal stromal cells, MSCs) and apheresis-derived suspension cells (i.e., peripheral blood mononuclear cells, PBMCs) were described to demonstrate how to prepare the cells (e.g., culture and harvest MSCs, isolated PBMCs) and subsequently execute automated and controlled process development procedures that are amenable to cell therapy product manufacturing.
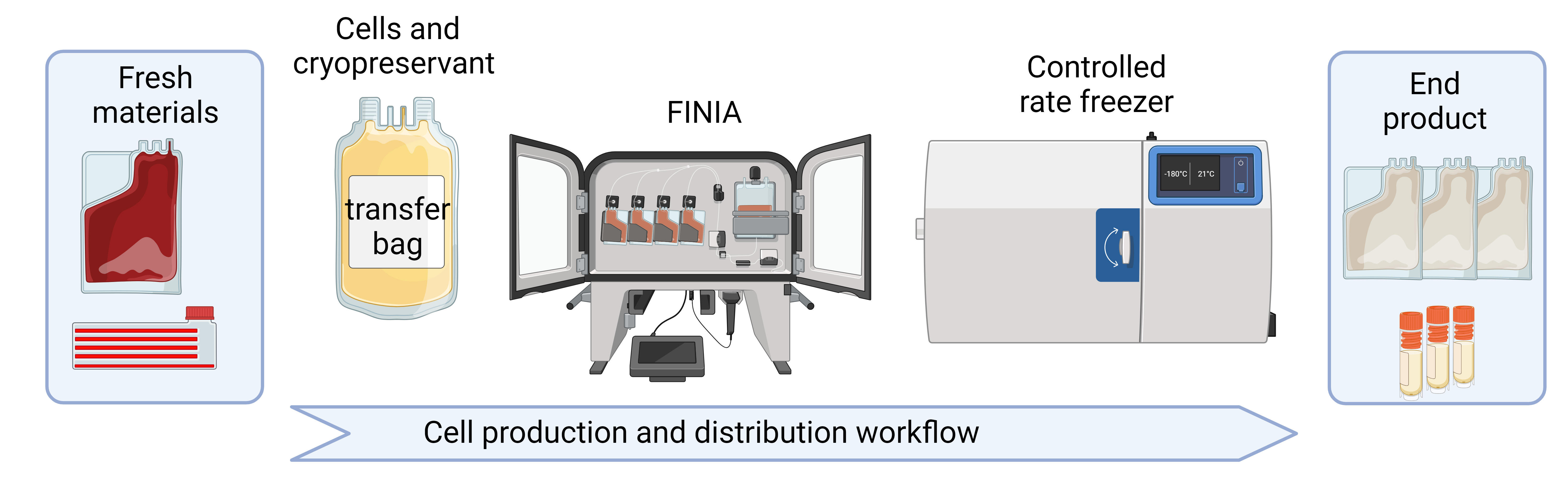
The Finia® Fill and Finish System (Terumo Blood and Cell Technologies) is an automated, closed system that offers temperature-controlled processing for formulation and aliquoting of liquids, including cell suspensions, in preparation for cryopreservation (Sethi and Cunningham, 2021; Algorri et al., 2022). The system mixes and cools up to three materials (e.g., cells, buffers, cryopreservation solutions) to a specified temperature. The system offers flexible procedure programming to allow the user to define workstream needs, from simple aliquoting of a cell source to the stepwise cooling of cell solution, a relevant buffer, and a cryopreservation solution of choice. The final cell product is then aliquoted into individual product bags and automatically sealed. The single-use disposable set is composed of product bags appropriate for freezing, thawing, and administration in the clinic, and a quality control bag for testing (Sethi and Cunningham, 2020; Cunningham et al., 2022). The disposable set is offered in two volume configurations, allowing for the freezing of volumes from 10 to 70 mL per bag. The Finia system is governed by the cell processing application (CPA), which is a secure server-based software (i.e., protected from access by unauthorized individuals) for procedure management and record keeping, capable of tracking performance and protocol development for current Good Manufacturing Practices (cGMP) compliance.
The Finia system and controlled-rate freezer are two separate automated systems that together provide a closed system workflow, which has several advantages over common cell culture practices, including a reproducible cryopreservation procedure with >90% post-thaw cell viability and the prevention of risks related to contamination and operator error. In addition, product bags can store considerably more cells than cryovials (Sethi and Cunningham, 2021). Sethi and Cunningham performed studies to compare a robust manual process to the Finia system (automated process) using T cells and found that the targeted product volumes were more accurate using the automated processing, and cell viability (comparing pre-formulation, post-formulation, and post-thaw) was comparable between the two processes (Sethi and Cunningham, 2021). Cell therapy manufacturing can be further automated by the use of programmable, multi-step-controlled rate freezers, which can standardize and record freezing procedures (Meneghel et al., 2020). Based on these previous studies, our group has implemented streamlined methods for using the Finia system and a controlled-rate freezer for processing and cryopreservation of both adherent and suspension cell types commonly used in cell manufacturing as cell therapy products or as starting materials, respectively. Terumo Blood and Cell Technologies has performed internal testing on the Finia system to demonstrate it can be used for both adherent and suspension cells (data on file). Furthermore, the use of the Finia system in a workstream for a homogenous population of T cells was recently reported (Cunningham et al., 2022). This is the first published report on the use of the Finia system with adherent cells (i.e., MSCs) as well as heterogeneous suspension cells (i.e., PBMCs). Furthermore, we provide quality control results (based on cell count, viability, and phenotyping), troubleshooting guidance, and considerations for GMP and Good Laboratory Practice (GLP) compliance with application to automated and controlled process development procedures amenable to cell therapy product manufacturing.
Materials and reagents
Biological materials
Human MSCs derived from umbilical cord tissue cryovial, stored LN2 vapor phase (supplied by Duke University MC3)
Human peripheral blood one-tenth leukopak fresh (STEMCELL Technologies, 200-0092)
Reagents
Prime-XV MSC Expansion (XSFM) (Irvine Scientific, catalog number: 91149)
Penicillin/streptomycin (P/S) (Millipore Sigma, catalog number: P4333)
Phosphate buffered saline (PBS) Ca2+, Mg2+ free (Millipore Sigma, catalog number: TMS-012-A)
PLTGold® human platelet lysate (hPL) (Millipore Sigma, catalog number: SCM151;)
TrypLE Express 1× (Millipore Sigma, catalog number: 12605028)
Cryostor® CS-10 (Fisher Scientific, catalog number: NC9930384)
LymphoprepTM (STEMCELL Technologies, catalog number: 07801)
Fetal bovine serum (FBS) (any vendor)
Zombie UV Fixable Viability kit (BioLegend®, catalog number: 423107)
Human TruStain FcXTM (BioLegend®, catalog number: 422302)
BD CytofixTM fixation buffer (BD Biosciences, catalog number: 554655)
Solutions
Dilution buffer (see Recipes)
FC buffer (see Recipes)
Viability staining solution (see Recipes)
Fc block solution (see Recipes)
Recipes
Dilution buffer
Reagent Final concentration Quantity PBS Ca2+/Mg2+ free (1×) 98% 98 mL hPL 2% 2 mL Total 100% 100 mL FC buffer
Reagent Final concentration Quantity PBS Ca2+/Mg2+ free (1×) 98% 98 mL FBS 2% 2 mL Total 100% 100 mL Viability staining solution
Reagent Final concentration Quantity PBS Ca2+/Mg2+ free (1×) 99.999% 9.99 mL Zombie UV dye (reconstituted) 0.001% 10 μL Total 100% 10 mL Fc block solution
Reagent Final concentration Quantity FC buffer 90% 9 mL Human TruStain FcX 10% 1 mL Total 100% 10 mL
Laboratory supplies
CellBIND® surface HYPERFlask® cell culture vessel (Corning®, catalog number: 10020)
50 mL conical tubes (any vendor)
Storage bottle, sterile (any vendor)
Serological pipettes and tips, assorted volumes (any vendor)
Micropipettes and tips, P10–P1000 μL (any vendor)
T-150 transfer bag (Fisher Scientific, catalog number: NC0470433)
FINIA 50 tubing set (Terumo Blood and Cell Technologies, catalog number: 22050)
Note: The tubing set comprises a mixing bag, a QC bag (10–40 mL), and three storage bags (10–84 mL total).
FINIA 250 tubing set (Terumo Blood and Cell Technologies; catalog number: 22250)
Note: The tubing set comprises a mixing bag, a QC bag (10–40 mL), and three storage bags (29–210 mL total).
Vapor phase storage cryovials for cell samples including QC (any vendor)
Microcentrifuge tubes low binding (any vendor)
Via-1-CassetteTM cartridges (Chemometec, catalog number: 941-0012)
Syringe 10 mL with luer lok (any vendor)
Blunt fill needle 18-gauge length 1.5 inch (any vendor)
Controlled rate freezer canister rack adjustable (Thermo Fisher Scientific, catalog number: 11679084)
Stainless steel freezing canister with bag capacity 250 mL (Thermo Fisher Scientific, catalog number: 4000335)
Frame to hold canisters in LN2 storage (Thermo Fisher Scientific, catalog number: 4R5461)
Tank of liquid nitrogen (any vendor)
96-well plates (any vendor)
Equipment
Biosafety cabinet (BSC) level A2 (e.g., Thermo Fisher Scientific, catalog number: 1377)
Incubator set to 5% CO2 and 37 °C (e.g., HeraCell VIOS; Thermo Fisher Scientific)
Cell counter (e.g., Chemometec, model: NucleoCounter NC-200TM)
Note: Cell counting methods can vary. It is important to use a reliable and reproducible method for acquiring cell counts and diameter.
Refrigerated centrifuge capable of reaching 800 rcf/g and using 50 mL conical tubes (any vendor)
Micropipette P10–1000 μL (any vendor)
Pipette controller (any vendor)
Inverted microscope (e.g., Thermo Fisher Scientific, model: EVOS M5000)
Note: Microscope needs to be used for phase contrast imaging of cells only.
Finia Fill and Finish System (Terumo Blood and Cell Technologies)
Sterile tubing welder (Terumo Blood and Cell Technologies, model: TSCD-II)
Tubing sealer (Terumo Blood and Cell Technologies, model: T-SEAL Mobile)
Flow cytometer, e.g., CytoFlex (Beckman Coulter), LSRFortessa (BD)
Liquid nitrogen (LN2) dewar or cryo unit (any vendor)
Controlled rate freezer (e.g., Thermo Fisher Scientific, CryoMed, catalog number: 7454)
Note: Freezer needs to be programmable to -90 °C.
Software and datasets
Cell Processing Application (CPA) software version 2.1 with Finia Fill and Finish System
Flow cytometry software for data analysis, e.g., FlowJoTM software (BD Biosciences), CytoBank (Beckman Coulter)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Li, Y., Stevens, H. Y., Sivaraman, S., Porter, L. N., Hoffman, A. R., Gibb, S. L., Selvam, S. and Bowles-Welch, A. C. (2023). Streamlined Methods for Processing and Cryopreservation of Cell Therapy Products Using Automated Systems. Bio-protocol 13(24): e4900. DOI: 10.21769/BioProtoc.4900.
分类
细胞生物学 > 细胞分离和培养
细胞生物学 > 细胞分离和培养 > 细胞分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link