- EN - English
- CN - 中文
An Improved Protocol for the Matrigel Duplex Assay: A Method to Measure Retinal Angiogenesis
Matrigel Duplex分析的改进方案: 测量视网膜血管生成的方法
(*contributed equally to this work) 发布: 2023年12月05日第13卷第23期 DOI: 10.21769/BioProtoc.4899 浏览次数: 1889
评审: Vivien J. Coulson-ThomasTarsis Gesteira FerreiraSudhir Verma
Abstract
Neovascular diseases of the retina, such as diabetic retinopathy (DR) and age-related macular degeneration (AMD), are proliferative retinopathies involving the growth of new blood vessels on the retina, which in turn causes impairment and potential loss of vision. A drawback of conventional angiogenesis assays is that they are not representative of the angiogenic processes in the retina. In the retina, the new blood vessels grow (from pre-existing blood vessels) and migrate into a non-perfused region of the eye including the inner limiting membrane of the retina and the vitreous, both of which contribute to vision loss. The Matrigel Duplex Assay (MDA) measures the migration of angiogenic capillaries from a primary Matrigel layer to a secondary Matrigel layer, which resembles the pathological angiogenesis in AMD and DR. The methodology of MDA is comprised of two steps. In the first step, the human retinal microvascular endothelial cells (HRMECs) are mixed with phenol red–containing Matrigel (in a 1:1 ratio) and seeded in the center of an 8-well chamber slide. After 24 h, a second layer of phenol red–free Matrigel is overlaid over the first layer. Over the course of the next 24 h, the HRMECs invade from the primary Matrigel layer to the secondary layer. Subsequently, the angiogenic sprouts are visualized by brightfield phase contrast microscopy and quantified by ImageJ software. The present manuscript measures the angiogenesis-inhibitory activity of the Src kinase inhibitor PP2 in primary HRMECs using the MDA. The MDA may be used for multiple applications like screening anti-angiogenic drugs, measuring the pro-angiogenic activity of growth factors, and elucidating signaling pathways underlying retinal angiogenesis in normal and disease states.
Graphical overview
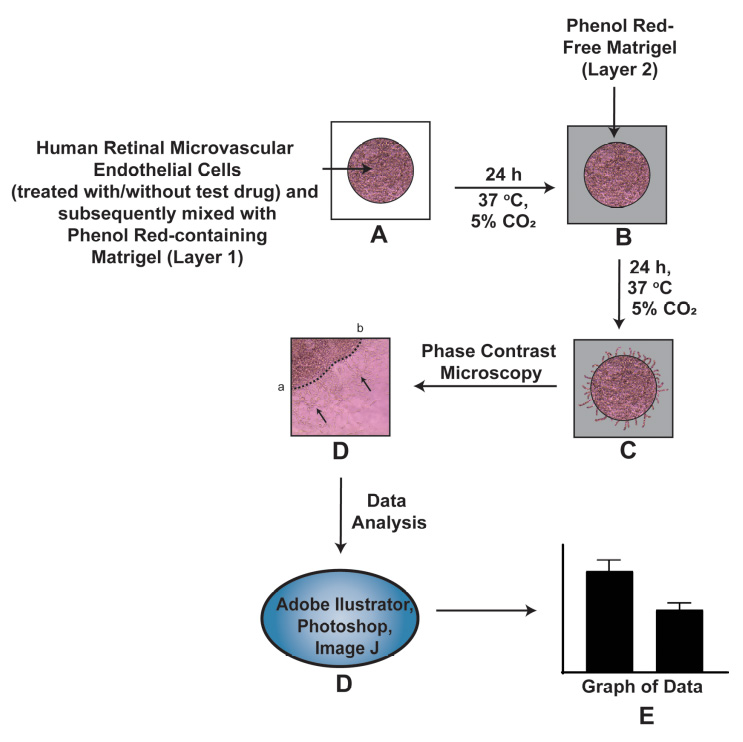
Background
The retina consists of organized layers of photoreceptors, interneurons, glia, epithelial cells, and endothelial cells. The proper maintenance of vascular networks is critical to normal visual function [1]. Aberrant angiogenesis is the hallmark of several ocular diseases including age-related macular degeneration (AMD) and diabetic retinopathy (DR) [2]. The angiogenic process in the retina is a complex, multistep process involving endothelial cell invasion, adhesion, chemotactic migration, proliferation, and differentiation into capillary tube–like structures and the production of a basement membrane around the vessel [3]. A survey of literature shows that the Matrigel capillary tube assay is one of the most prevalent angiogenesis assays in cell culture models [4, 5]. In this assay, human microvascular endothelial cells are seeded on a three-dimensional layer of solidified Matrigel (or any other reconstituted basement membrane extracellular matrix). Over a period of 24 h, the cells differentiate into capillary tube–like networks, which can be quantified by digital image analysis. A caveat of this assay is that it does not represent pathological angiogenesis in the eye. The Matrigel assay is more representative of vasculogenesis, which is defined as the differentiation of endothelial cells to yield de novo primitive vascular networks rather than angiogenesis where new capillaries are generated from existing vasculature [6]. Another disadvantage is the lack of a lumen in the capillaries obtained by this assay [7, 8]. Other methods used to measure retinal angiogenesis include the measurement of endothelial cell proliferation or endothelial cell chemotaxis or migration. Although these individual processes are useful indicators of angiogenic activity, they do not provide a holistic representation of the angiogenic cascade [7, 8]. These drawbacks are circumvented by using the Matrigel Duplex Assay (MDA). In the MDA, the angiogenic sprouting (in the secondary Matrigel layer) arises from pre-formed vascular networks in the primary layer [9, 10].
The MDA represents a combination of all the steps of retinal angiogenesis and provides a highly relevant model for the study of pro- and anti-angiogenic agents in vitro. In-depth morphological studies have shown that the angiogenic sprouting (occurring in the MDA) closely mimics retinal angiogenesis in vivo. Electron microscopy studies have demonstrated the presence of a lumen and elaborate cell–cell junctions within the endothelial cell aggregates observed in the MDA [9, 10]. As the endothelial capillary tube–like structures invade into the secondary Matrigel layer, the leading edge of the capillary sprouts is associated with long filopodia. The structure of these filopodia resembles those seen at the angiogenic front during developmental angiogenesis in the neonatal retina [9]. The retinal endothelial cells grown within the two layers of Matrigel reflect the biological characteristics of the neovascular retina, such as diffusion gradient of oxygen, nutrients, and pH. The growth of the cells inside the Matrigel duplex system allows for complex cell–cell and cell–matrix interaction. Furthermore, these retinal endothelial sprouts may be characterized and quantified by confocal microscopy [9].
Stitt et al. (2005) were the first to report the protocol for the MDA to study retinal angiogenesis in diabetic retinopathy [10]. During the application of the original protocol of Stitt et al. (2005) in our laboratory, some technical challenges occurred. The original protocol uses phenol red–containing Matrigel for both the primary and the secondary Matrigel layers [9, 10]. We observed that the interface between the two layers was difficult to visualize by phase contrast microscopy. This is especially true when there is a dense network of retinal capillaries migrating from the primary to the secondary Matrigel layer in the MDA. In order to circumvent this issue, we used a black Sharpie marker pen (in our early studies) to draw a boundary encircling the first layer before adding the second Matrigel layer [11]. This approach had its limitations as it was difficult to accurately demarcate the boundary of the first layer. Secondly, the black boundary around the primary layer appeared as a thick black band under the brightfield microscope, increasing the difficulty of accurately visualizing and quantifying the angiogenic capillary sprouts in the second layer. We drew the dotted line in the center of this black band to quantify the invasion of endothelial capillary networks into the secondary layer [11] and the whole process became arduous and cumbersome.
We devised a novel strategy to clearly differentiate between the two Matrigel layers in the MDA by utilizing phenol red–free Matrigel (PR-free-Matrigel) and phenol red–containing Matrigel (PR-Matrigel) for the individual layers. Initially, we tried to use PR-free-Matrigel for the primary layer and PR-Matrigel for the secondary layer but found that this arrangement resulted in poor quality photographs under a microscope. Subsequently, we reversed the layers (using the PR-Matrigel in the primary layer and PR-free-Matrigel in the secondary layer) and obtained clearer pictures under phase contrast microscopy, providing readily reproducible images and assay results. The present manuscript describes the ability of the Src Kinase inhibitor PP2 [12] (at a concentration of 10 µM) to suppress retinal angiogenesis of human retinal microvascular endothelial cells (HRMECs) using our modified MDA protocol. We hope that the MDA will be a useful tool for researchers working in the field of physiological and pathological angiogenesis in the retina.
Materials and reagents
Primary human retinal microvascular endothelial cells (HRMECs) (Cell Systems, catalog number: ACBRI 181). Grow HRMECs in endothelial growth media-2 (EGM-2) [EGMTM-2 Endothelial Cell Growth Medium-2 BulletKitTM (Lonza, catalog number: CC-3162)]. The BulletKitTM contains EBM-2 basal medium (catalog number: CC-3156) and SingleQuots growth factor supplements (catalog number: CC-4176) needed for the growth of HRMECs. The BulletKitTM also contains a vial of antibiotic (Gentamycin) and fetal bovine serum (FBS). Store the EBM-2 at 4 °C and the SingleQuots and FBS at -20 °C. Prepare the EGM-2 media following the manufacturer’s instructions provided in the BulletKitTM. Add FBS to the media at a final concentration of 2% (see Recipes). The EGM-2 medium with 2% FBS will be hereafter referred to as EGM-2 complete medium
RPMI-1640 medium (ATCC, catalog number 30-2001)
Trypsin-EDTA (0.25% Trypsin, 0.53 mM EDTA) (ATCC, catalog number: 30-2101). Long-term storage at -20 °C; however, aliquot Trypsin-EDTA and store at 4 °C for immediate use
Dulbecco’s Phosphate buffered saline (DPBS) without calcium and magnesium (Corning, Fisher, catalog number: 21-031-CM). Store at 4 °C
Absolute anhydrous ethanol (200 proof) (Pharmaco, Greenfield Global, catalog number: 111000200). Dilute ethanol with distilled water to obtain a 70% ethanol solution for wiping down the laminar flow hood
PR-Matrigel membrane matrix (Fisher, catalog number: CB-40234). Aliquot the Matrigel in Corning sterile freezing vials and store at -70 °C for long-term storage. Before the experiment, keep the Matrigel aliquot overnight at 4 °C (in a refrigerator) for thawing. During the assay, Matrigel should be kept on ice throughout the experiment
PR-free-Matrigel membrane matrix (Fisher, catalog number: CB-40234C)
PP2 (Enzo Biosciences, catalog number: 50-201-0681). PP2 is a potent and selective inhibitor of the Src family tyrosine kinases). Store PP2 at -20 °C and solubilize in DMSO (Fisher, catalog number: MT-25950CQC) to yield the stock solution at concentration of 10 mM (see Recipes). Aliquot the PP2 stock solution into microcentrifuge tubes and store at -20 °C. Immediately before use, thaw and dilute the PP2 in EBM-2 basal media (without FBS or growth factors) to a working concentration of 10 µM. After use, discard the remaining solution of 10 µM PP2
Tissue culture plasticware and related supplies
Corning cell counting chamber (Fisher, catalog number: 07-200-988)
Nunc Lab-Tek 8-well Permanox plastic chamber slide system (Fisher, catalog number: 12-565-22)
Corning BioCoat Collagen-coated T-25 (Fisher, catalog number: 08-774-327) and T-75 (Fisher, catalog number: 08-774-327) tissue culture flask. The BioCoat flasks are stored at 4 °C
Corning sterile cryogenic vials (Fisher, catalog number: 13-700-504)
Sterile polypropylene centrifuge tubes, 50 mL (Fisher, catalog number: 06-443-20)
Sterile polypropylene centrifuge tubes, 15 mL (Fisher, catalog number: 05-539-5)
Safety vented wash bottles (1,000 mL) for storing 70% ethanol (Fisher, catalog number: 11-865-170)
Microcentrifuge tubes, 1.5 mL (Fisher, catalog number: 05-408-129). Autoclave the microcentrifuge tubes before using them for the MDA
Sterile 5 mL microcentrifuge tube (Eppendorf, catalog number: 0030119487)
Sterile plastic serological pipettes (individually Wrapped, paper peel packaging, plugged):
25 mL pipette (Fisher, catalog number: 12-567-604)
10 mL pipette (Fisher, catalog number: 12-567-603)
5 mL pipette (Fisher, catalog number: 12-567-602)
2 mL pipette (Fisher, catalog number: 12-567-601)
1 mL pipette (Fisher, catalog number: 12-567-600)
Fisherbrand 5 3/4 inches’ disposable borosilicate glass Pasteur pipettes (Fisher, catalog number: 13-678-20B). Autoclave the Pasteur pipettes before using them for the MDA
Fisherbrand sterile high precision #22 style scalpel blade (Fisher, catalog number: 12-000-161)
DrummondTM Portable Pipet-AidTM XP pipette Controller (Fisher, catalog number: 13-681-15E)
PIPETMAN® 4-Pipette kit P2, P20, P200, P1000 (Gilson, catalog number: F167360)
Fisherbrand SureOne sterile aerosol barrier tips (100–1,000 µL) (Fisher, catalog number: 02-707-404)
Fisherbrand SureOne sterile aerosol barrier tips (20–200 µL) (Fisher, catalog number: 02-707-430)
Fisherbrand SureOne sterile aerosol barrier tips (0.1–20 µL) (Fisher, catalog number: 02-707-470)
Other consumable laboratory supplies
Labcoat (Fisher, catalog number: 19-181-596)
Ansell MICROFLEXTM NeoProTM NEC-288 Neoprene small size (Fisher, catalog number: 19-350-340F)
and medium size gloves (Fisher, catalog number: 19-350-340A)
Freezer boxes for long-term storage of Matrigel and PP2 (Cole-Parmer Essentials 81-Place Freezer Boxes, catalog number: UX-04396-63)
Kimtech Kimwipe delicate task wipers (Fisher, catalog number: 06-666A)
Sterile nonwoven gauze sponges 4 × 4 inch (Fisher, catalog number: 22-028-558)
Hayman style microspatula (Fisher, catalog number: 21-401-25A)
Solutions
EGM-2 complete medium (see Recipes)
EGM-2 media (with growth factors) (see Recipes)
10 mM PP2 (see Recipes)
200 µM PP2 (see Recipes)
VEH-20X (see Recipes)
Recipes
EGM-2 complete medium
The EGMTM-2 Endothelial Cell Growth Medium-2 BulletKitTM contains the following ingredients:
1× EBM-2 basal medium (CC-3156), 500 mL
1× EGM-2 SingleQuots supplement pack (CC-4176) containing:
1× white cap vial with VEGF, 0.50 mL
1× gray cap vial with hFGF-B, 2 mL
1× yellow cap vial with R3-IGF-1, 0.50 mL
1× green cap vial with hEGF, 0.50 mL
1× orange cap vial with heparin, 0.50 mL
1× blue cap vial with ascorbic acid, 0.50 mL
1× red cap vial with GA-1000, 0.50 mL
1× natural cap vial with hydrocortisone, 0.20 mL
1× bottle FBS, 10 mL
Please use the following table to prepare the required volume of EGM-2 complete medium:
EGM-2 complete media (mL) VEGF (µL) FGF (µL) IGF-1 (µL) EGF (µL) Heparin (µL) Ascorbic acid (µL) Gentamycin (GA-1000) (µL) Hydrocortisone (µL) FBS (mL) 50 50 200 50 50 50 50 50 20 1 100 100 400 100 100 100 100 100 40 2 250 250 1,000 250 250 250 250 250 100 5 400 400 1,600 400 400 400 400 400 160 8 500 500 2,000 500 500 500 500 500 200 10
EGM-2 media (with growth factors)
We usually make EGM-2 media (with growth factors) in a bottle and add the FBS to the T-75 flask directly. This is because we often do experiments in serum-free conditions, so we add the FBS separately during cell culture as required.
10 mM PP2
Molecular weight of PP2 = 301.78
Weigh 6 mg of PP2 in a sterile microcentrifuge tube. In the laminar flow hood, dissolve the PP2 in 2 mL of DMSO. Vortex briefly to obtain 10 mM PP2 stock solution. Aliquot this PP2 stock solution (as 50 µL aliquots) into microcentrifuge tubes and store at -20 °C.
200 µM PP2
Thaw out one aliquot of PP2 (concentration = 10 mM) in a water bath (held at 37 °C). Add 2 µL of PP2 in 100 µL of basal EBM-2 medium. Vortex vigorously. Now the concentration of PP2 is 200 µM (called PP2-20X in the text). Use this PP2 solution as described in Section D, Step 8. Discard the remaining solution of PP2-20X.
VEH-20X
Add 2 µL of DMSO in 100 µL of basal EBM-2 medium. Vortex vigorously to obtain VEH-20X solution (the concentration of DMSO in this solution is 2% v/v), which is referred to as VEH-20X. Use the VEH-20X solution as described in Section D, Step 10. Discard the remaining solution of VEH-20X.
Equipment
NU-540 (LabGard® ES NU-540 class II, type A2) laminar-flow biosafety cabinet (NuAire, Plymouth, MN, catalog number: NU-540)
Cell culture incubator (Heracell VIOS 150i cell culture incubator) (Thermo Scientific, Waltham, MA, catalog number: 51-032-872)
Leica DM IL LED inverted phase contrast microscope with camera (VWR International, catalog number: 76382-982)
Fisher Isotemp 220A water bath (Fisher, catalog number: FSGPD05)
Thermo Electron Corporation IEC Centra CL2 benchtop centrifuge (Thermo Scientific, catalog number: 004260F)
Microbalance (Sartorius, Model 1712 MP8, silver edition, catalog number: CUBIS_II_ANALYTICAL)
Vortex mixer (Fisher, catalog number: 02-215-414)
Software
LAS Image Capture Software (Leica Microsystems)
Adobe Photoshop 2023 (Adobe Creative Cloud for Windows)
Adobe Illustrator 2023 (Adobe Creative Cloud for Windows)
NIH ImageJ Version 1.47 (National Institutes of Health, Bethesda)
GraphPad Prism Version 9
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Brown, K. C., Light, R. S., Modi, K. J., Conely, K. B., Sugrue, A. M., Cox, A. J., Miles, S. L., Valentovic, M. A. and Dasgupta, P. (2023). An Improved Protocol for the Matrigel Duplex Assay: A Method to Measure Retinal Angiogenesis. Bio-protocol 13(23): e4899. DOI: 10.21769/BioProtoc.4899.
分类
细胞生物学 > 组织分析
发育生物学 > 细胞生长和命运决定 > 血管生成
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link