- EN - English
- CN - 中文
Human Schwann Cells in vitro II. Passaging, Purification, Banking, and Labeling of Established Cultures
体外人雪旺细胞 II. 已建立培养物的传代、纯化、储存和标记
发布: 2023年11月20日第13卷第22期 DOI: 10.21769/BioProtoc.4882 浏览次数: 2327
评审: Vivien J. Coulson-ThomasDjamel Eddine ChafaiRomina Vuono
Abstract
This manuscript describes step-by-step procedures to establish and manage fresh and cryopreserved cultures of nerve-derived human Schwann cells (hSCs) at the desired scale. Adaptable protocols are provided to propagate hSC cultures through serial passaging and perform routine manipulations such as enzymatic dissociation, purification, cryogenic preservation, live-cell labeling, and gene delivery. Expanded hSCs cultures are metabolically active, proliferative, and phenotypically stable for at least three consecutive passages. Cell yields are expected to be variable as determined by the rate of growth of individual batches and the rounds of subculture. The purity, however, can be maintained high at >95% hSC regardless of passage. The cells obtained in this manner are suitable for various applications, including small drug screens, in vitro modeling of neurodevelopmental processes, and cell transplantation. One caveat of this protocol is that continued expansion of same-batch hSC populations is eventually restricted due to senescence-linked growth arrest.
Keywords: Ensheathing glia (鞘外胶质细胞)Background
Human Schwann cells (hSCs) from nerve tissues are fairly amenable cells for in vitro culture. Whereas preparing primary cells requires labor-intensive procedural steps, standard, general practices can be applied to manage the cultures successfully once the hSCs are established. hSCs are expandable under adherent conditions. Substantial scaling up of same-prep cultures can be achieved by supplementing the culture medium with specific mitogenic factors and plating the cells on matrix-coated dishes, preferably with laminin [reviewed in Monje (2020a and 2020b)].
The steady expansion of individual hSC populations is feasible up to the second or third passage because the rate of hSC proliferation diminishes thereafter in most preparations. However, a single harvest of adult nerve-derived fascicles may yield cultures able to expand at a >103 amplification rate and total yields may surpass 100 million cells within two or three rounds of subculture. Established hSC cultures maintain key characteristics of lineage-committed SCs, as evidenced by the expression of SC-specific markers such as NGFR, S100B, Nestin, and Sox10 (Peng et al., 2020). Aberrant growth or spontaneous hSC transformation has not been observed (Emery et al., 1999). Indeed, hSCs from expanded batches are deemed safe for transplantation partly due to their phenotypic stability and resistance to transform (Bastidas et al., 2017). In managing established hSC cultures, researchers should be mindful that the cells achieve an irreversible state of growth arrest or senescence by or around the fifth passage, or the equivalent of 20 population doublings, after their initial isolation from an adult nerve source (Levi et al., 1995; Levi, 1996).
This protocol describes our optimized approaches to propagate hSC cultures and perform various routine manipulations in vitro. A thorough description of materials, methods and procedures is presented here to obtain scalable hSC cultures from both fresh isolates and cryogenic stocks. The banking of hSCs is described in detail due to its multiple benefits for deferred experimentation, long-term storage, and transfer of cell cultures. Several purification and cell labeling methods are introduced along with recommendations on choosing certain methods for specific applications. The following sections describe step-by-step procedures to facilitate replication in other labs. Figure 1 presents a generic representation of suggested interventions at any given step of the culture workflow. This paper does not include comprehensive data sets. The microscopy image data in the figures and videos are provided for qualitative purposes only. Our publications contain more information about laboratory-scale experimentation using established hSC cultures (Monje et al., 2018; Peng et al., 2020). Investigators can use our papers to gather additional details on experimental design, use of positive and negative controls, and analysis of results from diverse assays. The hSC cultures prepared as described herein are reliable models for the development of cell-based platforms, including reconstituted co-culture systems, and studies of xenotransplantation. Lastly, these methodologies are intended for basic research, since they differ substantially from clinical protocols (Khan et al., 2021), and may be effective only when using hSC cultures prepared from normal nerve fascicles or ganglia. We have not used hSC cultures from unconventional sources.
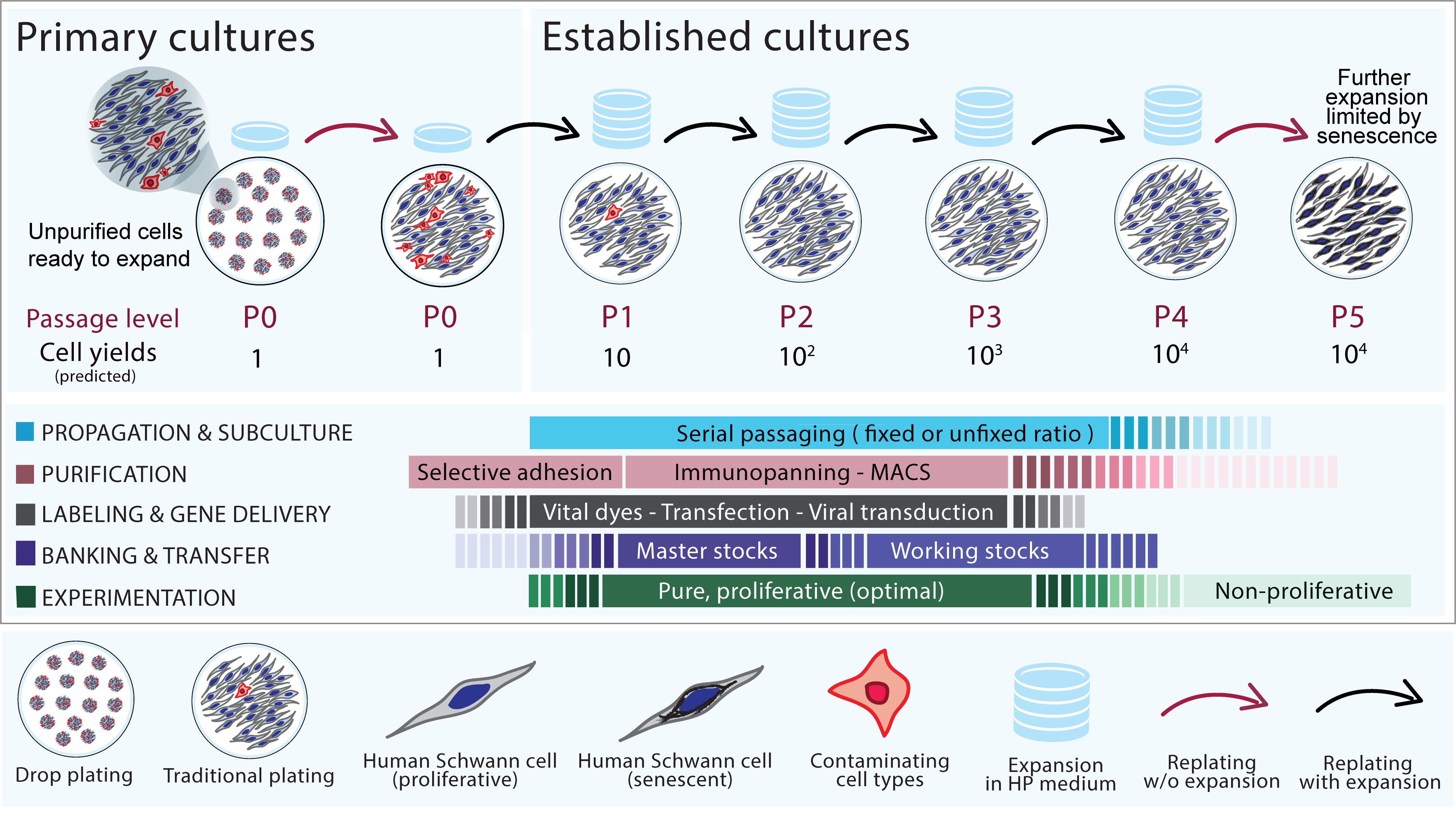
Figure 1. Generation and management of established human Schwann cells (hSC) cultures: passaging, purification, transfer, and in vitro modifications.The diagram summarizes the suggested steps described in each protocol. Primary, passage-zero (P0) hSC cultures (drop-plated dish, left panel) can be expanded at a 1:10 ratio (controlled passaging, upper panel) or an unfixed ratio (routine passaging) until enough cells are obtained or the cultures become senescent, as indicated. Purification, banking, and gene delivery (or labeling) can be attempted at any level of passage but preferably no later than passage-2 or -3, which is the optimal time for experimentation using cells that are both proliferative and pure. The indicated cell yields represent accumulated cell numbers in each passage as predicted by the expansion rate of a typical hSC culture containing 1 million cells at P0.
Materials and reagents
All materials, reagents, and solutions should be sterile and cell culture grade. If possible, consistently use materials and reagents of the same brand and maintain a record of lot numbers in case signs of cell toxicity are observed. The list below is not intended to be fully comprehensive or limit the use of products from certain manufacturers. The product information is provided for reference only. Follow the manufacturer’s recommendations and use best practices in cell culture to avoid microbial contamination and ensure the reproducibility of the results.
Supplies and consumables
Disposable serological pipettes (5, 10, and 25 mL), polystyrene, plugged, sterile, and individually wrapped (VWR, catalog numbers: 76201-710, 75816-100, and 75816-090)
Polystyrene Pasteur (transfer) pipettes, sterile and individually wrapped (VWR, Argos Technology, catalog number: 10122-560)
Petri dish, bacteriological grade, 10 cm, not tissue culture treated (Corning, catalog number: 351029)
Centrifuge tubes, 15 and 50 mL, polypropylene, conical-bottom (Corning, catalog numbers: 430791 and 430290)
Cell culture dishes (35, 60, and 100 mm), polystyrene, tissue culture treated (Corning, catalog numbers: 353001, 353002, and 353003)
Multi-well plates (6, 12, and 24-well), tissue culture treated, flat bottom (Corning, catalog numbers: 3506 and 3524)
Cell culture flasks (T25 and T75), canted neck with plug seal caps (Corning, catalog numbers: 430168 and 430720U)
Cryogenic vials, 2 mL, internal thread (Corning, catalog number: 430489)
Polycarbonate freezing container with a tube holder, Nalgene “Mr. Frosty” Freezing Container (VWR, catalog number: 55710-200)
Cell lifter, individually wrapped, polyethylene, 2 cm length blade, 18 cm long (VWR, catalog number: T-2443-4)
Ice pans (VWR, catalog number: 89233) containing wet ice and dry ice
Media, supplements, and other cell culture products
Distilled water, cell culture grade (Fisher Scientific, Gibco, catalog number: 15-230-147)
Hank’s balanced salt solution (HBSS), formulated without calcium or magnesium and containing phenol red, pH 7.2 (Thermo Fisher Scientific, Gibco, catalog number: 14170-112)
Leibovitz’s L15 Medium (L15) (Thermo Fisher Scientific, Gibco, catalog number: 11415064)
Dulbecco’s Modified Eagle’s Medium (DMEM), with high glucose and phenol red, pH 7.2 (Thermo Fisher Scientific, Gibco, catalog number: 11965092). (Optional) Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12) (Thermo Fisher Scientific, Gibco, catalog number: 11320033)
Gentamycin 1,000× provided as 50 mg/mL stock solution (Thermo Fisher Scientific, Gibco, catalog number: 15750-060). (Optional) Penicillin-Streptomycin, 10,000 U/mL stock solution (Thermo Fisher Scientific, Gibco, catalog number: 15140-148)
De-complemented fetal bovine serum (FBS) (HyClone, catalog number: SV 30014.03). Stored in aliquots at -80 °C; used in all applications requiring serum, including cryopreservation of cell stocks
GlutaMAX supplement (100×) consisting of 200 mM L-alanyl-L-glutamine dipeptide in 0.85% NaCl (Thermo Fisher Scientific, Gibco, catalog number: 35050061)
Heregulin-β1, 177-244 amino acid peptide (Preprotech, catalog number: G-100-03); instructions on the preparation, storage, and use of heregulin-β1 stock solution (25 µM) can be found in our protocol Andersen and Monje (2018). The stock solution is best preserved when kept in aliquots at -80 °C and used only for media preparation, avoiding freezing/thawing cycles. Heregulin-β1 is the primary mitogenic factor for hSCs; it is referred to as “heregulin” in the text and figures
Forskolin powder (Sigma-Aldrich, catalog number: F68861); instructions on the preparation, storage, and use of forskolin stock solution (15 mM) can be found in our protocol Andersen and Monje (2018). Keep the forskolin stock solution in the -80 °C freezer for long-term preservation of the cAMP-inducing activity. Forskolin is a synergistic enhancer of heregulin-dependent hSC proliferation
0.5% Trypsin/EDTA (TE) solution (10×), without phenol red (Thermo Fisher Scientific, Gibco, catalog number: 15400054). (Optional) TrypLETM select enzyme, 1× (Thermo Fisher Scientific, Gibco, catalog number: 12563011)
Laminin stock, consisting of a sterile 1 mg/mL laminin solution from Engelbreth-Holm-Swarm murine sarcoma basement membrane (Sigma-Aldrich, catalog number: L2020), stored at -80 °C in aliquots for single use. Prepare laminin-coated dishes as described in Andersen and Monje (2018). Always use freshly coated laminin dishes for seeding hSCs. Nerve fibroblasts can be plated in regular cell culture–treated dishes without coating
Poly-L-lysine (PLL) powder (Sigma, catalog number: P-263). Prepare a PLL stock solution and use it for coating dishes as described in Andersen and Monje (2018). Store air-dried PLL-coated dishes at 4 °C for up to one month
Dulbecco’s phosphate-buffered saline (DPBS), pH 7.2 (Thermo Fisher Scientific, Gibco, catalog number: 14190)
Dimethyl sulfoxide (DMSO) (Invitrogen, catalog number: D12345). Used as a component of the freezing medium and solvent of lyophilized fluorophores
(Optional) RecoveryTM cell culture freezing medium, ready to use, stored in aliquots at -80 °C (Thermo Fisher Scientific, Gibco, catalog number: 12648010)
Isopropyl alcohol (Fisher Chemical, catalog number: A416P-4L)
Tris base, powder (Thermo Fisher Scientific, Invitrogen, catalog number: 15504020)
Hydrochloric acid (HCl) solution, 1 N, bioreagent suitable for cell culture (Sigma, catalog number: 7647-01-0); used to balance pH in buffers and cell culture medium
Antibodies, dyes, and assorted commercially available products
Anti-NGFR mouse IgG monoclonal antibody, produced from the HB-8737 hybridoma cell line (American Type Culture Collection, ATCC). See Ravelo et al. (2018) for technical details on our culture protocols for this and other hybridoma cell lines
Anti-O4 mouse IgM monoclonal antibody, produced from the O4 hybridoma cell line (Sommer and Schachner, 1981), kindly provided by Dr. Melitta Schachner. (Optional) Use purified O4 antibodies from a commercial source (Novus Biologicals, catalog number: NL637)
Anti-mouse immunoglobulins, goat polyclonal antibody (anti-IgG, IgA, and IgM), affinity purified, liquid, unconjugated (ICN/Cappel, catalog number: 55486)
CellTrackerTM Green CMFDA (5-chloromethylfluorescein diacetate) powder (Invitrogen, Molecular Probes, catalog number: C7025)
(Optional) Pluronic F-127 (Molecular Probes, catalog number: P-6866)
Hoeschst-34580 (Molecular probes, catalog number: H21486) prepared in water at 1 mg/mL
Basic NucleofectorTM kit for primary mammalian glial cells (Lonza, catalog number: VPI-1006)
GFP plasmids. The pmaxGFPTM expression vector (0.5 µg/µL in 10 mM Tris pH 8.0) can be used as provided in the Basic NucleofectorTM kit or expanded in house
Lentiviral particles, aliquoted and stored at -80 °C for up to six months. For a reference, we have used lentiviral expression vectors for the fluorescent reporters EGFP and mCherry (Monje et al., 2018) acquired as ultra-purified, ready-to-use, packaged lentiviruses from the Viral Vector Core Facility, The Miami Project to Cure Paralysis, Miami, FL. Follow the manufacturer’s recommendations on the safe storage and use of viral stocks, including the MOI determination for each virus lot. Multiple freeze/thaw cycles of the viral particles are not recommended
(Optional) Polybrene infection/transfection reagent, 10 mg/mL stock solution (Santa Cruz Biotechnology, catalog number: sc-134220)
(Optional) Eukaryotic antibiotics for the selection of virally-infected cells. Puromycin dihydrochloride (Santa Cruz Biotechnology, catalog number: sc-108071); Blasticidin S HCl solution (Santa Cruz Biotechnology, catalog number: sc-495389); Hygromycin B solution (Santa Cruz Biotechnology, catalog number: sc-29067)
Solutions
Low proliferation medium (LP) (see Recipes)
High proliferation medium (HP) (see Recipes)
TE dissociation solution (see Recipes)
Freezing medium (see Recipes)
Immunoglobulins solution (see Recipes)
Equipment
Biosafety cabinet, BL2 level (Thermo Fisher Scientific, model: 1300 Series A2)
CO2 cell incubator set up at 37 °C and 8%–9% CO2 (Thermo Fisher Scientific, model: Forma Steri-Cycle)
Benchtop centrifuge (Beckman Coulter, model: Allegra X-I2R)
Inverted phase contrast microscope with an attached digital camera (VWR, model: V5MP)
Cell counter for automated counting of cells in suspension (Bio-Rad, TC20). (Optional) Hemocytometer for manual cell counting
4 °C refrigerator and -80 °C laboratory freezer (Thermo Fisher Scientific, model: RLE Series)
Liquid nitrogen storage tank (Thermo Fisher Scientific, model: Locator JR Plus)
Single cuvette-based NucleofectorTM I device and certified cuvettes (formerly Amaxa Biosystems, now Lonza)
Inverted fluorescence microscope equipped with a UV, FITC, and TRITC filter sets (Olympus IX71) and attached digital camera
(Optional) Live-cell imaging system IncuCyte ZOOMTM (Essen BioScience, MI) for time-lapse microscopy of cultured cells plated in multi-well dishes
Software
(Optional) IncuCyte Zoom 2015A, Rev 1 for image capture and video assembly of time-lapse microscopy data
Image analysis software, ImageJ-FIJI (NIH), free-access software available at https://imagej.net/software/fiji/. Used to estimate the covered area (confluency) in selected phase contrast images using the area measuring tool
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Monje, P. V. (2023). Human Schwann Cells in vitro II. Passaging, Purification, Banking, and Labeling of Established Cultures. Bio-protocol 13(22): e4882. DOI: 10.21769/BioProtoc.4882.
分类
神经科学 > 周围神经系统 > 施万细胞
细胞生物学 > 细胞分离和培养 > 细胞生长
细胞生物学 > 细胞分离和培养 > 低温贮存
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link