- EN - English
- CN - 中文
Analysis of Plasmodium falciparum Mitochondrial Electron Transport Chain Activity Using Seahorse XFe96 Extracellular Flux Assays
利用海马XFe96细胞外通量测定法分析恶性疟原虫线粒体电子传递链的活性
发布: 2023年11月05日第13卷第21期 DOI: 10.21769/BioProtoc.4863 浏览次数: 2444
评审: Alka MehraHangjun Ke

相关实验方案

TZA, 一种基于敏感报告因子细胞学检测方法, 可用于准确快速量化静息CD4+ T细胞中可诱导的且复制能力强的潜伏HV-1
Anwesha Sanyal [...] Phalguni Gupta
2019年05月20日 7794 阅读

使用 Seahorse XFe96 细胞外通量分析仪实时分析弓形虫寄生虫的线粒体电子传递链功能
Jenni A. Hayward [...] Giel G. van Dooren
2022年01月05日 4173 阅读
Abstract
The mitochondrial electron transport chain (ETC) is a multi-component pathway that mediates the transfer of electrons from metabolic reactions that occur in the mitochondrion to molecular oxygen (O2). The ETC contributes to numerous cellular processes, including the generation of cellular ATP through oxidative phosphorylation, serving as an electron sink for metabolic pathways such as de novo pyrimidine biosynthesis and for maintaining mitochondrial membrane potential. Proper functioning of the mitochondrial ETC is necessary for the growth and survival of apicomplexan parasites including Plasmodium falciparum, a causative agent of malaria. The mitochondrial ETC of P. falciparum is an attractive target for antimalarial drugs, due to its essentiality and its differences from the mammalian ETC. To identify novel P. falciparum ETC inhibitors, we have established a real-time assay to assess ETC function, which we describe here. This approach measures the O2 consumption rate (OCR) of permeabilized P. falciparum parasites using a Seahorse XFe96 flux analyzer and can be used to screen compound libraries for the identification of ETC inhibitors and, in part, to determine the targets of those inhibitors.
Key features
• With this protocol, the effects of candidate inhibitors on mitochondrial O2 consumption in permeabilized asexual P. falciparum parasites can be tested in real time.
• Through the sequential injection of inhibitors and substrates into the assay, the molecular targets of candidate inhibitors in the ETC can, in part, be determined.
• The assay is applicable for both drug discovery approaches and enquiries into a fundamental aspect of parasite mitochondrial biology.
Graphical overview
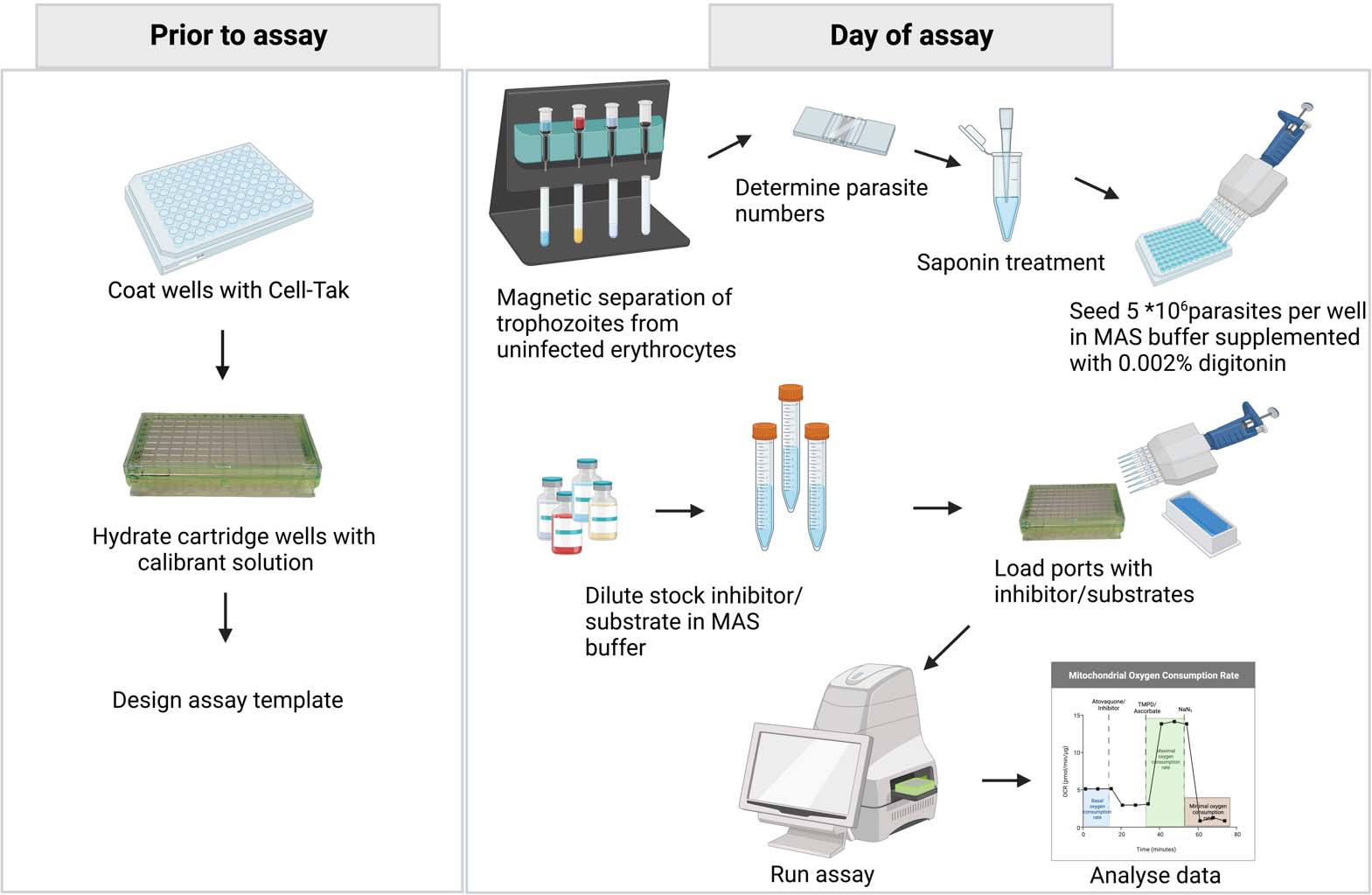
Seahorse assay experimental workflow. Prior to the assay, coat the cell culture microplate with Cell-Tak to help adhere the parasites to the wells; hydrate the cartridge wells to ensure proper sensor functionality and design the assay template using the Agilent Seahorse Wave Desktop software (Analyze Seahorse data files, Seahorse Wave desktop software|Agilent). On the day of the assay, prepare the inhibitors/substrates that are to be injected into the ports. Then, separate 3 × 108 trophozoite-stage parasites from the uninfected red blood cells (RBCs) and ring-stage parasites using a MACS® magnetic column. Check the purity of the parasites with Giemsa-stained smears. Determine the concentration of infected RBCs in the sample using a hemocytometer and dilute to approximately 5 × 107 parasites per milliliter. Treat infected RBCs with saponin to permeabilize the host cell membrane and seed approximately 5 × 106 parasites (100 μL) per well in mitochondria assay solution (MAS) buffer. Supplement MAS buffer with digitonin to permeabilize the parasite plasma membrane. Load the ports with the prepared inhibitors/substrates and run the assay using a Seahorse XFe96 analyzer. Once the assay is completed, analyze the data using the Wave desktop software. Further data processing can be done using statistical analysis software.
Background
Plasmodium species cause malaria in humans and were responsible for 247 million malaria cases and 619,000 deaths in 2021 (WHO report, 2022). The emergence of parasites resistant to frontline antimalarial drugs has contributed to the increase in malaria cases and deaths in recent years (Conrad and Rosenthal, 2019; Ippolito et al., 2021). Hence, it is important to look beyond the currently available antimalarial drugs for new treatments. The mitochondrion of Plasmodium is a validated drug target, and some common antimalarial compounds interfere with its electron transport chain (ETC) (Hikosaka et al., 2015; Ke and Mather, 2017).
The ETC is located in the inner mitochondrial membrane of eukaryotes and facilitates the transfer of electrons through a series of redox centers to O2. Electrons are sourced from the oxidation of substrates such as malate, succinate, NADH, glycerol 3-phosphate, and dihydroorotate in the mitochondrion (Hayward and van Dooren, 2019). These electrons are donated to coenzyme Q (CoQ). Reduced CoQ docks at the cytochrome bc1 complex (also known as Complex III). Complex III facilitates the transfer of electrons to an electron carrying protein, cytochrome c (CytC). From CytC, electrons are donated to the cytochrome c oxidase complex (Complex IV), where the reduction of O2, the final electron acceptor of the ETC, occurs. The transfer of electrons through Complexes III and IV is coupled to the net movement of protons from the mitochondrial matrix to the mitochondrial intermembrane space (Figure 1). The proton motive force that is established across the inner mitochondrial membrane by the ETC is critical for the synthesis of ATP by the ATP synthase complex (Figure 1).
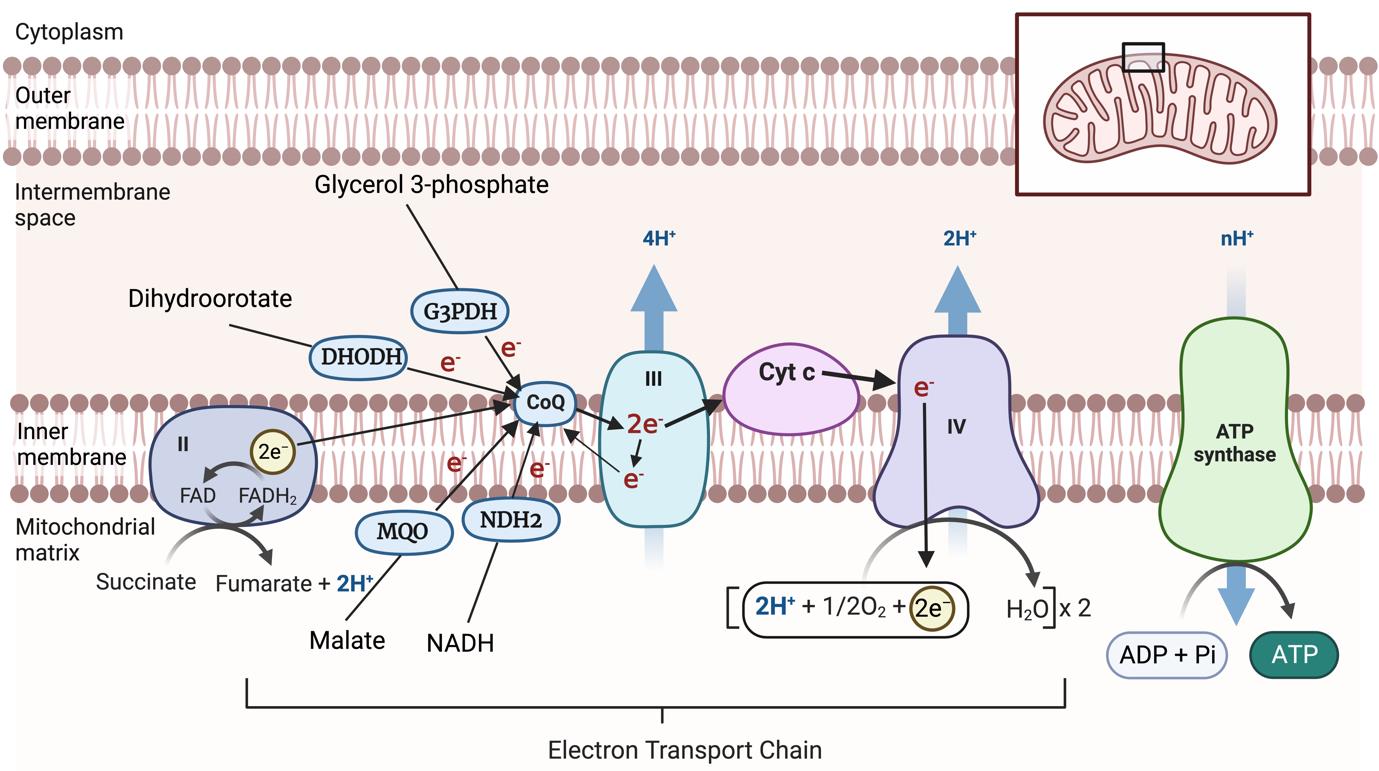
Figure 1. Mitochondrial electron transport chain. Electrons from the oxidation of a range of different substrates in the mitochondrion are donated to coenzyme Q (CoQ). CoQ donates these electrons to Complex III, from where they are donated via cytochrome c (CytC) to Complex IV, where electrons ultimately reduce O2 to form H2O. Electron transport events are indicated by arrows. The passage of electrons through Complexes III and IV is coupled to the net transport of protons (H+) from the mitochondrial matrix to the intermembrane space, generating an H+ gradient that can be harnessed by ATP synthase to generate ATP. DHODH, dihydroorotate dehydrogenase; G3PDH, glycerol 3-phosphate dehydrogenase; MQO, malate: quinone oxidoreductase; NDH2, type II-NADH dehydrogenase; FADH2, Dihydroflavine-adenine dinucleotide; FAD, Flavin adenine dinucleotide. The inset indicates the position of the enlarged portion within the mitochondrion. Created with BioRender.com.
The P. falciparum ETC varies considerably from the ETC of their mammalian hosts (Hayward and van Dooren, 2019). For example, the P. falciparum ETC has a single subunit “type II” NADH dehydrogenase instead of the multi-subunit Complex I found in mammals and a malate:quinone oxidoreductase enzyme that feeds electrons from malate oxidation directly into the ETC (Figure 1; Rajaram et al., 2022). Complex III is a validated drug target in apicomplexans, and the compounds that inhibit Complex III often target either (or in rare cases both) of the CoQ binding sites in the complex. Structural differences in the CoQ binding sites of parasite Complex III compared with human Complex III likely account for the high degree of selectivity of inhibitors like atovaquone against the parasite complex (Srivastava et al., 1999; Fisher et al., 2020). Mutations in the CoQ binding sites of parasite Complex III can confer clinically relevant levels of resistance against compounds like atovaquone (Staines et al., 2018). It is therefore of interest to identify novel inhibitors of Complex III or the other components of the P. falciparum ETC, ideally ones that remain potent against parasites already resistant to existing ETC inhibitors.
Measuring cellular O2 consumption is a powerful means of assessing ETC function. We have recently developed a suite of Seahorse XFe96 flux analyzer assays to measure mitochondrial O2 consumption rates in the apicomplexan parasite Toxoplasma gondii, a species from the same phylum as Plasmodium (Hayward et al., 2022). We have now established similar assays to enable the measurement of mitochondrial O2 consumption rates in permeabilized asexual-stage Plasmodium falciparum parasites and describe them in this protocol (Graphical overview; General note 1). The assay can be used to screen compound libraries to identify ETC inhibitors in P. falciparum. The assay can aid in determining the molecular target of identified inhibitors in the ETC and the effectiveness of novel inhibitors against drug-resistant parasite strains (Hayward et al., 2023; Nguyen et al., 2023). The assay can also be used to obtain molecular insights into the functionality of the P. falciparum ETC.
Materials and reagents
175 cm2 tissue culture flasks (Sarstedt, catalog number: 83.3912)
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: H3375-250G)
5% (w/v) digitonin (Thermo Fisher Scientific, InvitrogenTM, catalog number: BN 20061)
Albumax II (Gibco, catalog number: 11021-045-1kg)
Atovaquone (Sigma-Aldrich, catalog number: A7986-10MG)
Cell-Tak (Corning, catalog number: 354241)
D-glucose (Sigma-Aldrich, catalog number: G7021)
D-mannitol (Sigma-Aldrich, catalog number: M9546-250G)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418-250G)
Ethylene glycol-bis(2-amino-ethylether)-N,N,N,N’-tetra acetic acid (EGTA) (Sigma-Aldrich, catalog number: E3889-25G)
Fatty acid-free bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A3803-100G)
General plastic consumables [sterile Eppendorf tubes, sterile falcon tubes (15, 50 mL), sterile pipette tips (20, 200, and 1,000 μL), sterile serological pipettes (10, 25 mL); Sarstedt or Corning]
Gentamicin (Invitrogen, catalog number: 15710-072)
Hypoxanthine (Sigma-Aldrich, catalog number: H-9636)
L-ascorbic acid (Sigma-Aldrich, catalog number: A5960-25G)
L-malic acid (Sigma-Aldrich, catalog number: M1000-100G)
Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M2670-100g)
Milli-Q water (fresh from any available purification system)
N,N,N,N’-tetramethyl-p-phenylenediamine (TMPD) (Sigma-Aldrich, catalog number: T7394-5G)
Potassium chloride (Sigma-Aldrich, catalog number: 7447-40-7, 500G)
Potassium phosphate monobasic (KH2PO4) (ChemSupply, catalog number: PA009-500G)
RPMI-HEPES with Glutamax (Thermo Fisher ScientificTM, catalog number: 72400120)
Sodium azide (AJAX-Finechem Univar, catalog number: AJA1222-100G)
Sodium chloride (Sigma-Aldrich, catalog number: S5886-5KG)
Sodium dihydrogen phosphate monohydrate (AnalaR, catalog number: 102454R)
Sorbitol (Sigma-Aldrich, catalog number: 50-70-4)
Sucrose (Sigma-Aldrich, catalog number: S9378-5KG)
Ascorbate stock (200 mM) (see Recipes)
Albumax II (5% w/v) (see Recipes)
Atovaquone stock (25 mM) (see Recipes)
Complete culture medium (see Recipes)
Malate stock (500 mM) (see Recipes)
MAS buffer (3× stock) (see Recipes)
MAS buffer (1× working solution) (see Recipes)
Phosphate buffered saline (10×) (see Recipes)
Saponin (0.15% w/v) (see Recipes)
Sodium azide stock (100 mM) (see Recipes)
Sodium bicarbonate (0.1 M) (see Recipes)
Sorbitol (5% w/v) (see Recipes)
TMPD stock (500 mM) (see Recipes)
Biological material
Plasmodium falciparum cells (wild type 3D7)
Recipes
Ascorbate stock (200 mM)
Dissolve 352 mg of L-ascorbic acid in 5 mL of 1× MAS buffer. Adjust pH to 7.4 with potassium hydroxide (KOH) and adjust final volume with 1× MAS buffer to 10 mL. Aliquot ascorbate stock (500 μL/aliquot) and store at -20 °C for long-term storage.
Albumax II (5% w/v)
Dissolve 25 g of Albumax II in 500 mL of RPMI medium. Filter sterilize using the Millipore Stericup Vacuum Driven Disposable Filtration System. Aliquot Albumax II stock (50 mL/aliquot) and store at -20 °C for long-term storage.
Atovaquone stock (25 mM)
Dissolve 10 mg of atovaquone in 1.09 mL of DMSO. Aliquot atovaquone stock (50 μL/aliquot) and store at -20 °C for long-term storage.
Complete culture medium
Prepare complete culture medium from RPMI 1640-HEPES with Glutamax supplemented with the cell culture media components shown below. Store complete culture medium at 4 °C and pre-warm to 37 °C before use.
Components Final concentration Volume RPMI medium - 500 mL Additional glucose (2.5 M) 10 mM 2 mL Hypoxanthine (200 mM) 480 μM 1.2 mL Gentamicin (10 mg/mL) 20 μg/mL 1 mL 5% Albumax solution 0.375% w/v 37.5 mL Heat-inactivated human serum 2.5% v/v 12.5 mL Malate stock (500 mM)
Dissolve 670.45 mg of L-malic acid in 5 mL of MAS buffer. Adjust pH to 7.4 with KOH and adjust the final volume with MAS buffer to 10 mL. Aliquot malate stock (1.5 mL/aliquot) and store at -20 °C for long-term storage.
MAS buffer (3× stock)
Weigh out the components (listed below) and dissolve in 50–75 mL of Milli-Q water by placing the solution into a water bath and/or use a magnetic stirrer. Adjust pH of the solution to 7.4 using KOH. Bring the final volume to 125 mL. Filter sterilize for long-term storage.
Components Final concentration Weight Mannitol 660 mM 15.03 g Sucrose 210 mM 8.99 g KH2PO4 30 mM 0.51 g MgCl2 15 mM 0.381 g HEPES 6 mM 0.1785 g EGTA 3 mM 0.1425 g MAS buffer (1× working solution)
On the day of the experiment, mix 15 mL of 3× MAS buffer with 30 mL of Milli-Q water, to get 45 mL of 1× MAS buffer. Add 90 mg of fatty acid-free BSA and 2.25 mL of 0.5 M malate.
Phosphate buffered saline (10×)
Components Weight NaCl 80 g KCl 2 g Na2HPO4 14.4 g KH2PO4 1.9 g Dissolve the compounds in 800 mL of Milli-Q water. Adjust pH of the solution to 7.4 using HCl. Bring the final volume to 1 L. Sterilize the solution by autoclaving for 20 min at 15 psi. Store at room temperature. When needed, mix 100 mL of 10× PBS with 900 mL of Milli-Q water to get 1× PBS.
Saponin (0.15% w/v)
Dissolve 0.15 g of saponin in 100 mL of PBS. Filter-sterilize the solution using a 0.2 μm filter. Store at 4 °C for long-term storage. When needed, mix 1 mL of 0.15% saponin with 2 mL of PBS to obtain a 0.05% (w/v) saponin working solution.
Sodium azide stock (100 mM)
Dissolve 65.1 mg of sodium azide in 10 mL of MAS buffer. Aliquot sodium azide stock (500 μL/aliquot) and store at -20 °C for long-term storage.
Sodium bicarbonate (0.1 M)
Dissolve 84 mg of sodium bicarbonate in approximately 8 mL of Milli-Q water. Adjust pH to 8.0 and make up the volume to 10 mL. Filter-sterilize using a 0.2 μm filter. Store at room temperature.
Sorbitol (5% w/v)
Dissolve 5 g of sorbitol in 100 mL of Milli-Q water. Filter-sterilize the solution using a 0.2 μm filter. Store at 4 °C for long-term storage.
TMPD stock (500 mM)
Dissolve 82.2 mg of TPMD in 1 mL of ethanol. Aliquot TMPD solution (50 μL/aliquot) and store at -20 °C for long-term storage.
Equipment
4 °C fridge and -20 °C freezer
Tabletop centrifuge for 15 and 50 mL tubes (Beckman Coulter, model: Allegra X-15R)
Tabletop centrifuge for microcentrifuge tubes (Thermo Scientific, model: Heraeus Pico 17)
Improved Neubauer hemocytometer (Hirschmann, model: 8100104)
Humidified 37 °C non-CO2 incubator
MACS® magnetic column (CS columns, Miltenyi Biotec, catalog number: 130-041-305) with a sterile 3-way stopclock
SuperMACS II separator (Miltenyi Biotec, catalog number: 130-044-104)
Multichannel pipettes (30–300 μL) (Gilson)
pH meter (Cyberscan pH510)
Pipettes (0.2–2, 2–20, 20–200, and 100–1,000 μL) (Gilson)
Reagent reservoirs
Seahorse XFe96 Analyzer (Agilent)
Seahorse XFe96 FluxPak (XFe96 cell culture microplate, XFe96 sensor cartridge and utility plate, XF Calibrant Solution) (Agilent, catalog number: 102416-100)
Standard inverted light microscope (Olympus CKX41), fitted with 20× objective lense
Biological safety cabinet (Safemate 1.2 vision, Edwards Group)
Water bath, 37 °C (WB7, Ratek)
Zip-lock bags
Software
Wave Desktop Software, version 2.6.3 (for running the Seahorse XFe96 assay)
GraphPad Prism 9 (for statistical analyses of the results)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Ramesh, S., Cihalova, D., Rajendran, E., van Dooren, G. G. and Maier, A. G. (2023). Analysis of Plasmodium falciparum Mitochondrial Electron Transport Chain Activity Using Seahorse XFe96 Extracellular Flux Assays. Bio-protocol 13(21): e4863. DOI: 10.21769/BioProtoc.4863.
分类
微生物学 > 微生物细胞生物学 > 基于细胞的分析方法 > 报告基因实验
细胞生物学 > 细胞新陈代谢 > 呼吸测量法
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










