- EN - English
- CN - 中文
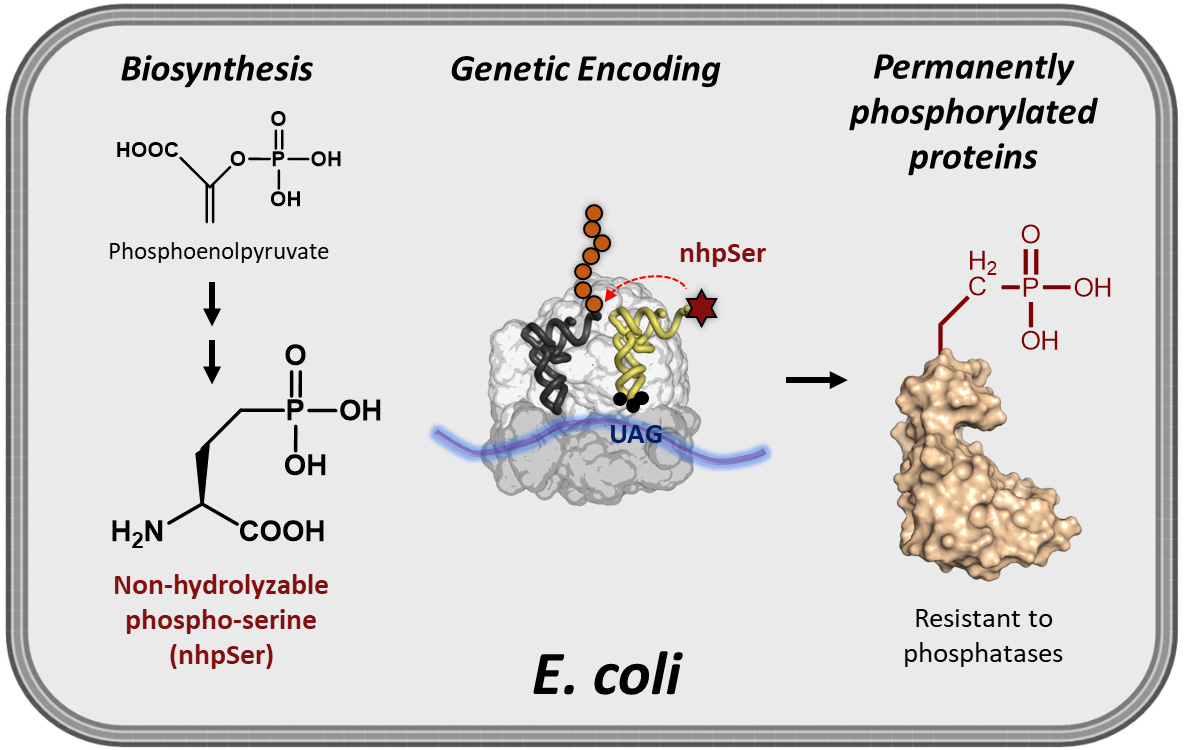
Biosynthesis and Genetic Encoding of Non-hydrolyzable Phosphoserine into Recombinant Proteins in Escherichia coli
大肠杆菌中非水解磷酸丝氨酸的生物合成和基因编码为重组蛋白
发布: 2023年11月05日第13卷第21期 DOI: 10.21769/BioProtoc.4861 浏览次数: 2496
评审: Willy R Carrasquel-UrsulaezBhanu JagilinkiAnonymous reviewer(s)
Abstract
While site-specific translational encoding of phosphoserine (pSer) into proteins in Escherichia coli via genetic code expansion (GCE) technologies has transformed our ability to study phospho-protein structure and function, recombinant phospho-proteins can be dephosphorylated during expression/purification, and their exposure to cellular-like environments such as cell lysates results in rapid reversion back to the non-phosphorylated form. To help overcome these challenges, we developed an efficient and scalable E. coli GCE expression system enabling site-specific incorporation of a non-hydrolyzable phosphoserine (nhpSer) mimic into proteins of interest. This nhpSer mimic, with the γ-oxygen of phosphoserine replaced by a methylene (CH2) group, is impervious to hydrolysis and recapitulates phosphoserine function even when phosphomimetics aspartate and glutamate do not. Key to this expression system is the co-expression of a Streptomyces biosynthetic pathway that converts the central metabolite phosphoenolpyruvate into non-hydrolyzable phosphoserine (nhpSer) amino acid, which provides a > 40-fold improvement in expression yields compared to media supplementation by increasing bioavailability of nhpSer and enables scalability of expressions. This “PermaPhos” expression system uses the E. coli BL21(DE3) ∆serC strain and three plasmids that express (i) the protein of interest, (ii) the GCE machinery for translational installation of nhpSer at UAG amber stop codons, and (iii) the Streptomyces nhpSer biosynthetic pathway. Successful expression requires efficient transformation of all three plasmids simultaneously into the expression host, and IPTG is used to induce expression of all components. Permanently phosphorylated proteins made in E. coli are particularly useful for discovering phosphorylation-dependent protein–protein interaction networks from cell lysates or transfected cells.
Key features
• Protocol builds on the nhpSer GCE system by Rogerson et al. (2015), but with a > 40-fold improvement in yields enabled by the nhpSer biosynthetic pathway.
• Protein expression uses standard Terrific Broth (TB) media and requires three days to complete.
• C-terminal purification tags on target protein are recommended to avoid co-purification of prematurely truncated protein with full-length nhpSer-containing protein.
• Phos-tag gel electrophoresis provides a convenient method to confirm accurate nhpSer encoding, as it can distinguish between non-phosphorylated, pSer- and nhpSer-containing variants.
Graphical overview
Background
Discerning molecular mechanisms by which phosphorylation alters protein structure and function requires efficient methods to make homogenously and site-specifically phosphorylated proteins in milligram quantities for in vitro characterization. Kinases can be used to install phospho-groups onto target proteins, but the complexities of kinase specificity and their activation hinder broad application of these approaches. Genetic code expansion (GCE), on the other hand, enables production of site-specifically phosphorylated proteins by installing phospho-amino acids into proteins during translation in response to UAG (amber) stop codons. Escherichia coli GCE systems for expressing proteins homogenously modified with one or more phosphoserine (pSer) groups are well developed and becoming more widely used (Rogerson et al., 2015; Zhu et al., 2019). However, pSer proteins produced in E. coli via these GCE encoding systems can become partially dephosphorylated during expression by a few phosphatases that E. coli does harbor, frustrating downstream applications. Further, experiments requiring exposure of purified phospho-proteins to environments with phosphatases will compromise their phosphorylation status. As one example where this is an issue, “pulldowns” are a time-tested technique to discover new protein–protein interactions whereby bait proteins are mixed into cell lysates and then retrieved along with any bound prey proteins, which can then be identified by mass spectrometry or immunological assays. A phosphorylated bait protein will be hydrolyzed back to the wild-type form by phosphatases when added to eukaryotic lysates, even in the presence of phosphatase inhibitors, so that the retrieved pool of prey proteins will be a mixture of wild-type and phospho-protein interactors (Zhu et al., 2023). Consequently, it is challenging (if not impossible) to distinguish between proteins that preferentially interact with the phosphorylated or the wild-type form of the prey protein.
A GCE system to encode a stable, non-hydrolyzable mimic of pSer called phosphono-methyl-alanine (also known as 2-amino-4-phosphono-butryate and here referred to as non-hydrolyzable pSer or “nhpSer” for simplicity) was developed by Chin and colleagues in 2015 (Rogerson et al., 2015). This system relies on adding exogenous nhpSer to the culture media while expressing the same amino-acyl tRNA synthetase (RS)/tRNA pair and EF-Tu variant (EF-Sep) used for pSer incorporation. To ensure nhpSer is encoded over endogenous pSer, an E. coli ΔserC mutant is used in combination with overexpression of SerB (Figure 1). The negatively charged nhpSer does not traverse the cellular membrane effectively when exogenously added to the culture media, and so we developed a 6-step biosynthetic pathway that converts phosphoenolpyruvate into nhpSer inside the cell (Figure 2) to increase bioavailability of nhpSer for subsequent encoding into a protein, providing > 40-fold improvement in nhpSer-protein production and enabling scalability of expressions (Zhu et al., 2023). This “PermaPhos” expression system provided us with sufficient quantities of nhpSer-containing proteins to confirm that nhpSer mimics pSer function in several cases where aspartate/glutamate do not (Zhu et al., 2023). We outline here the general workflow for expressing a control protein (super folder GFP, sfGFP) containing nhpSer at site N150, and confirming correct encoding. Strategies to adopt this protocol for biologically relevant proteins are discussed.
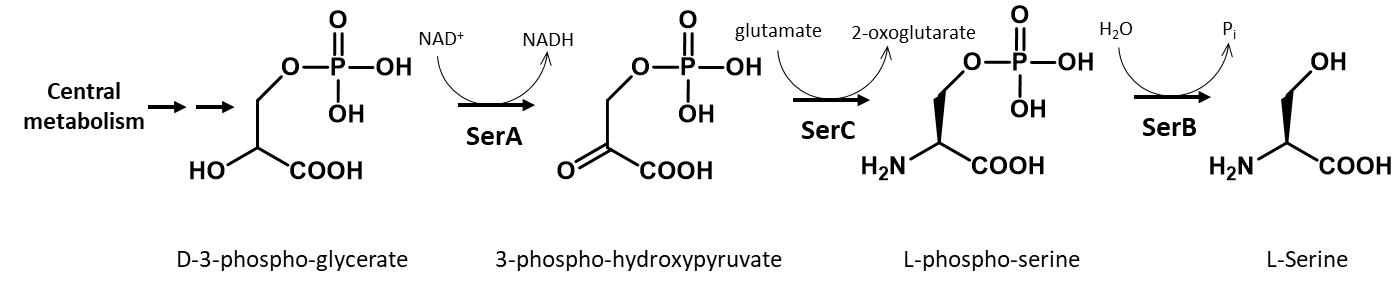
Figure 1. Biosynthesis of serine in E. coli. In this protocol, a ∆serC mutant expression host is used as it lacks the ability to biosynthesize pSer amino acid, which would compete with nhpSer encoding. SerB is also overexpressed to hydrolyze any free pSer amino acid that might enter the cell from the media or be formed by promiscuous transaminases that can substitute for SerC function. For GCE systems that encode authentic phosphoserine, ∆serB expression hosts are used in order to build up intracellular pSer concentrations.
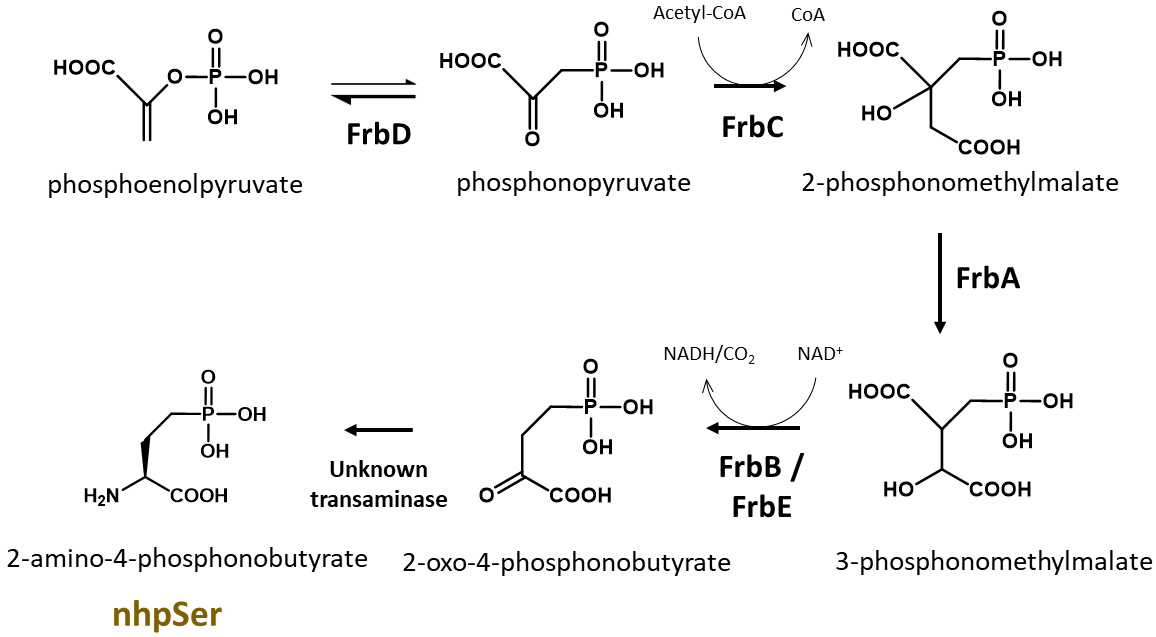
Figure 2. Biosynthesis of nhpSer amino acid by Streptomyces rubellomurinus FrbA, B, C, D, and E proteins. In this protocol, the pCDF-Frb-v1 plasmid expresses five Frb enzymes that, along with an unknown transaminase in E. coli, convert the central metabolite phosphoenolpyruvate into nhpSer, which is then encoded into target proteins using the GCE machinery.
Materials and reagents
Biological materials
Strains
BL21(DE3) ∆serC (Addgene, #197656). This BL21(DE3) strain of E. coli has the serC gene knocked out to prevent biosynthesis of phosphoserine, which would compete with nhpSer for incorporation into proteins (Figure 1). This strain contains Release Factor 1 (RF1), the protein responsible for terminating translation at TAG amber codons, so truncated protein will be produced along with full-length nhpSer-protein. To avoid co-purification of truncated protein with full-length protein, C-terminal purification tags are recommended. For proteins that self-assemble into homo-multimers (dimers, trimers, etc.), purification can be challenging due to the possible co-purification of truncated forms that are incorporated as subunits in the assembly. If using N-terminal purification/solubilization tags, additional purifications steps may be needed to remove truncated protein species. See General note 1 for more discussion.
DH10b (Thermo Fisher, catalog number: EC0113). This strain can be used for faithful propagation of plasmids and for cloning needs when users wish to clone their genes of interest into the pRBC plasmid (see Plasmids below). Do not use for protein expression. Though we have not explicitly tested all of them, other classical cloning strains of E. coli can be used including NEB 10-beta (New England BioLabs, catalog number: C3019H), DH5α (e.g., Thermo Fisher, catalog number: 18258012), NEB 5-alpha (New England BioLabs, catalog number: C2987H), or TOP10 (Thermo Fisher, catalog number: C404010).
Plasmids
pERM2-nhpSer (Addgene, #201922): machinery plasmid for nhpSer incorporation, kanamycin resistance with pUC origin of replication. This plasmid expresses the same tRNA synthetase and EF-Tu used for pSer incorporation (Rogerson et al., 2015). The amber codon suppressing Sep-tRNACUA is v2.0 developed by Chin and colleagues to minimize mis-aminoacylation (Zhang et al., 2017). The Sep-tRNA and tRNA-synthetase are constitutively expressed via lpp and GlnS promoters, respectively. The EF-Tu is under control of an IPTG inducible tac promoter. Also constitutively expressed via an OXB20 promoter is the E. coli serB protein to further eliminate (hydrolyze) free pSer amino acid that may come from the media or produced by promiscuous transaminases.
pCDF-Frb-v1 (Addgene, #201923): expresses Streptomyces rubellomurinus FrbA, FrbB, FrbC, FrbD, and FrbE enzymes required for the biosynthesis of nhpSer. See Zhu et al. (2023) for a detailed description of this biosynthetic pathway, which is also summarized in Figure 2. Each Frb protein is expressed under the control of an engineered T7 transcriptional promoter variant and so all Frb enzyme are expressed by the addition of IPTG. This plasmid confers spectinomycin resistance and contains the CloDF origin of replication. This plasmid is large (~11 kb) and contains repetitive elements; while we have not observed instability of this plasmid, given its large size it is good practice to minimize propagations. We recommend that when you receive the DH10b cells with the pCDF-Frb-v1 plasmid from Addgene, grow up a few individual colonies overnight in liquid media [e.g., 2× YT media supplemented with spectinomycin at 100 μg/mL (see Recipes)] and make frozen glycerol stocks of the cells for long-term storage. Glycerol stocks can be made by mixing 600 μL of overnight culture with 400 μL of sterile 50% (v/v) glycerol. Place culture tube(s) in -80 °C freezer for storage. These stocks serve as a permanent, long-term source of plasmid, which can be prepared by inoculating cultures with cells from a glob of the frozen glycerol stock. Do not thaw the glycerol stock(s) once frozen.
pRBC-sfGFP wild-type (Addgene, #174075): expresses wild-type sfGFP control protein with C-terminal His6 tag, under a T7 transcriptional promoter, ampicillin resistance/p15a origin of replication. sfGFP is expressed by the addition of IPTG.
pRBC-sfGFP 150TAG (Addgene, #174076): same as above except the sfGFP gene contains a TAG amber stop codon at site N150. The TAG codon is used to direct the translational encoding of nhpSer. Note that this is also the same plasmid used for pSer encoding (Zhu et al., 2022); any protein cloned into this vector for pSer encoding will also work for nhpSer encoding. See General note 2 for more discussions.
pRBC-[x] wild type (you must create): expresses your wild-type protein of interest (POI). You can clone your POI into pRBC by removing the sfGFP gene from the pRBC-sfGFP wt plasmid by restriction digest with NdeI and XhoI enzymes and replacing it with your POI gene using standard cloning techniques (e.g., ligation, Gibson Assembly, or SLiCE). For reasons mentioned above, a C-terminal purification tag is preferred. For helpful tips on construct design, see Troubleshooting 1.
pRBC-[x] TAG (you must create): expresses your POI with nhpSer encoded at a TAG codon. You can clone your POI into pRBC by removing the sfGFP gene by restriction digest with NdeI and XhoI and replacing with your POI gene using standard cloning techniques (e.g., ligation, Gibson Assembly, or SLiCE). Using site-directed mutagenesis, change the codon to TAG (the amber stop codon) where you intend to encode nhpSer into your POI.
Reagents
Tryptone (e.g., VWR, catalog number: 97063-386)
Yeast Extract (e.g., VWR, catalog number: 97064-368)
NaCl (e.g., VWR, catalog number: 97061-274)
Agar (e.g., VWR, catalog number: 97064-336)
MgSO4·7H2O (e.g., VWR, catalog number: 97062-134)
K2HPO4 (potassium dibasic) (e.g., VWR, catalog number: 97062-234)
KH2HPO4 (potassium monobasic) (e.g., VWR, catalog number: BDH9268)
α-D-glucose (e.g., VWR, catalog number: 97061-168)
Glycerol (e.g., VWR, catalog number: BDH24388.320)
Isopropyl b-D-1-thiogalactopyranoside (IPTG) (e.g., Anatrace, catalog number: I1003)
Ampicillin (e.g., VWR, catalog number: 97061-442)
Kanamycin (e.g., VWR, catalog number: 75856-684)
Spectinomycin (e.g., VWR, catalog number: 89156-368)
Antifoam B (e.g., J.T. Baker, catalog number: B531-05)
Phos-tag acrylamide (e.g., VWR, catalog number: 101974-086)
Dry ice
Ethanol (e.g., Sigma, catalog number: 65348-M)
Acrylamide/Bio-acrylamide solution (e.g., Bio-Rad, catalog number: 1610146)
Solutions
Phosphate Buffered Saline (PBS) (e.g., VWR, catalog number: 75800-982)
LB/agar (see Recipes)
2× YT media (see Recipes)
50% (v/v) glycerol (see Recipes)
10% (v/v) glycerol (see Recipes)
SOC media (see Recipes)
Starter culture media (see Recipes)
1.1× Terrific Broth media (see Recipes)
10× TB potassium phosphate buffer (see Recipes)
Ampicillin stock (see Recipes)
Kanamycin stock (see Recipes)
Spectinomycin stock (see Recipes)
0.5 M IPTG (see Recipes)
Recipes
LB/agar media (0.5 L)
Reagent Final concentration Quantity Tryptone 1% (w/v) 5 g Yeast extract 0.5% (w/v) 2.5 g NaCl 1.0% (w/v) 5 g Agar 1.5% (w/v) 7.5 g H2O n/a To 500 mL Total n/a 500 mL After mixing reagents thoroughly, autoclave on standard liquid setting to sterilize. Note the agar will not go into solution until autoclaved.
After autoclaving, gently swirl the bottle to ensure melted agar is evenly mixed.
Notes:
i. Store LB/agar bottle in a 55 °C oven and pour plates on an as needed basis. LB/agar can be stored in molten form for ~2 weeks if sterility is maintained.
ii. If an oven is not available, plates can be poured with antibiotics once LB/agar is sufficiently cooled to touch. Plates can be stored at 4 °C for up to a week.
2× YT media (1 L)
Reagent Final concentration Quantity Tryptone 1.6% (w/v) 16 g Yeast extract 1.0% (w/v) 10 g NaCl 0.5% (w/v) 5 g H2O n/a To 1,000 mL Total n/a 1 L After mixing reagents thoroughly, autoclave on the standard liquid setting to sterilize.
After autoclaving, allow to cool to room temperature before use.
50% (v/v) glycerol (500 mL)
Reagent Final concentration Quantity Glycerol (100%) 50% (v/v) 250 mL H2O n/a 250 mL Total n/a 500 mL Mix by placing a suitable magnetic stir bar in a 500 mL graduated cylinder and add 250 mL of water to graduated cylinder. While stirring, pour pure glycerol to the 500 mL mark on graduated cylinder. Stir for 5 min and then transfer to a 0.5 L bottle. Sterilize by autoclaving on liquid setting.
10% (v/v) glycerol (1 L)
Reagent Final concentration Quantity Glycerol (100%) 10% (v/v) 100 mL H2O n/a 900 mL Total n/a 1 L Mix by placing a suitable magnetic stir bar in a 1,000 mL graduated cylinder and add 900 mL of water to graduated cylinder. While stirring, pour pure glycerol to the 1,000 mL mark on graduated cylinder. Stir for 5 min and then transfer to a 1 L bottle. Sterilize by autoclaving on liquid setting.
SOC media, 50 mL
Reagent Final concentration Quantity 2× YT media n/a 49 mL 1 M MgSO4 10 mM 0.5 mL 40% (w/v) α-D-glucose 0.4% (w/v) or ~20 mM 0.5 mL Total n/a 50 mL 1 M MgSO4 can be made by mixing 12.3 g of MgSO4·7H2O in water up to 50 mL of total volume. Adjust mass of MgSO4 if using the salt with different hydration status.
40% (w/v) α-D-glucose can be made by mixing 20 g of α-D-glucose with water up to 50 mL of total volume. Mix thoroughly until glucose is dissolved. Gentle heating in a microwave may facilitate dissolution of glucose.
Sterilize MgSO4, glucose, and 2× YT solutions individually by autoclaving. Allow each component to cool to room temperature and mix as indicated above. Maintain sterility while adding components together.
It is easy to contaminate SOC. We suggest breaking this into 5 × 10 mL aliquots before use or making smaller batches. If sterility is maintained, SOC can be stored at room temperature indefinitely. It can also be stored at -20 °C but avoid repeated freeze/thaw.
Starter culture media: 2× YT + 0.5% (v/v) glycerol (50 mL)
Reagent Final concentration Quantity 2× YT media (sterile) n/a 49.5 mL 50% (v/v) glycerol (sterile) 0.5% (v/v) 0.5 mL Total n/a 50 mL Prepare immediately before use.
1.1× Terrific Broth media (0.9 L)
Reagent Final concentration (of 1×) Quantity Tryptone 1.2% (w/v) 12 g Yeast extract 2.4% (w/v) 24 g Glycerol (50% [v/v]) 0.5% (v/v) 10 mL H2O n/a To 900 mL Total n/a 900 mL Sterilize by autoclaving on liquid setting.
Do not mix TB phosphate buffer (below) until all solutions are cool and immediately before use.
10× TB potassium phosphate buffer (1 L)
Reagent Final concentration Quantity KH2PO4 (monobasic, anhydrous) 0.17 M 23.1 g K2HPO4 (dibasic, anhydrous) 0.72 M 125.4 g H2O n/a To 1 L Total n/a 1 L The masses provided for the potassium phosphate salts are for anhydrous formulations. Hydrate forms of these salts can be used, but masses must be adjusted to maintain indicated molarity.
Sterilize by autoclaving on liquid setting.
Do not mix TB phosphate buffer to 1.1× TB media until all solutions are sterilized and cooled to room temperature, and immediately before use.
Ampicillin stock (10 mL)
Reagent Final concentration Quantity Ampicillin 100 mg/mL 1 g H2O n/a To 10 mL Total n/a 10 mL Sterilize by filtering with a 0.2 μm syringe-end filter.
Store in 1 mL aliquots at -20 °C.
Kanamycin stock (10 mL)
Reagent Final concentration Quantity Kanamycin 50 mg/mL 0.5 g H2O n/a To 10 mL Total n/a 10 mL Sterilize by filtering with a 0.2 μm syringe-end filter.
Store in 1 mL aliquots at -20 °C.
Spectinomycin stock (10 mL)
Reagent Final concentration Quantity Spectinomycin 100 mg/mL 1 g H2O n/a To 10 mL Total n/a 10 mL Sterilize by filtering with a 0.2 μm syringe-end filter.
Store in 1 mL aliquots at -20 °C.
Note: Do not confuse spectinomycin with streptomycin. These antibiotics are not interchangeable.
0.5 M IPTG (10 mL)
Reagent Final concentration Quantity IPTG 0.5 M 1.19 g H2O n/a To 10 mL Total n/a 10 mL Sterilize by filtering with a 0.2 μm syringe-end filter.
Store in 1 mL aliquots at -20 °C.
Laboratory supplies
1.7 mL Eppendorf tubes (e.g., VWR, catalog number: 87003-294)
0.6 mL Eppendorf tubes (e.g., VWR, catalog number: 89000-010)
100 mm plates (e.g., VWR, catalog number: 470210-568)
0.5 L centrifuge bottles (must fit available centrifuges/rotor)
500 mL graduate cylinder
15 mL conical tubes (e.g., VWR, catalog number: 89126-798)
50 mL conical tubes (e.g., VWR, catalog number: 89039-656)
14 mL sterile culture tubes (e.g., VWR, catalog number: 60818-689)
250 mL baffled flasks (e.g., VWR, catalog number: 89095-266)
2.8 L baffled Fernbach flasks (e.g., VWR, catalog number: 22877-168)
Micro pipette tips 10 μL (e.g., VWR, catalog number: 76323-394)
Micro pipette tips 200 μL (e.g., VWR, catalog number: 76323-390)
Micro pipette tips 1,000 μL (e.g., VWR, catalog number: 76323-454)
0.2 μm syringe end filter (e.g., VWR, catalog number: 28145-477)
10 mL syringes (e.g., VWR, catalog number: 76124-664)
Equipment
Autoclave (any capable of sterilizing liquid media and culturing materials at 121 °C, and of a saturated steam pressure of 15 PSI)
Expression equipment:
Static incubator for growing LB/agar plates (set to 37 °C) (e.g., VWR, catalog number: 97025-630)
Shaker incubator for growing liquid cultures (e.g., New Brunswick I26R, Eppendorf, catalog number: M1324-0004)
i. Shaker should be able to rotate at 200–250 rpm
ii. Refrigeration is necessary for expressions below room temperature (< 25 °C)
iii. Shaker deck should have clamps to hold 250 mL and 2.8 L Fernbach flasks
Optical density 600 nm spectrophotometer (e.g., Ultrospec 10, Biochrome, catalog number: 80-2116-30)
Competent cell preparation and cell harvesting
Centrifuge capable of speeds of at least 5,000× g and able to hold volumes commensurate with culture sizes (e.g., Beckman Coulter, model: Allegra 25R)
Centrifuge bottles that can withstand centrifugal forces and hold the volume of cultures grown for harvesting cells. The type of bottles depends on the centrifuge and rotor used
Freezer (-80 °C), if cells are to be stored before protein is purified (e.g., VIP ECO ULT freezer, VWR, catalog number: 76305-596)
Electroporator (e.g., Eppendorf Eporator, Fisher Scientific, catalog number: E4309000027)
0.1 cm electro-cuvettes (e.g., Bio-Rad, catalog number:1652089)
Fluorometer capable of reading sfGFP fluorescence (excitation 488 nm/emission 512 nm). Handheld fluorometers work well for routine fluorescence reads (e.g., Turner Biosystems, model: PicoFluor)
-20 °C freezer for storing plasmids and antibiotics (e.g., Fisher Scientific, catalog number: 10-549-264)
Ice machine (e.g., Fisher Scientific, catalog number: 09-540-003)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Zhu, P., Mehl, R. A. and Cooley, R. B. (2023). Biosynthesis and Genetic Encoding of Non-hydrolyzable Phosphoserine into Recombinant Proteins in Escherichia coli. Bio-protocol 13(21): e4861. DOI: 10.21769/BioProtoc.4861.
分类
生物工程 > 合成生物学
生物化学 > 蛋白质 > 表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link