- EN - English
- CN - 中文
A Plate Growth Assay to Quantify Embryonic Root Development of Zea mays
量化玉米胚根发育的平板生长测定
发布: 2023年10月20日第13卷第20期 DOI: 10.21769/BioProtoc.4858 浏览次数: 2255
评审: Samik BhattacharyaErin SparksAntony Chettoor
Abstract
Murashige-Skoog medium solutions have been used in a variety of plant plate growth assays, yet most research uses Arabidopsis thaliana as the study organism. For larger seeds such as maize (Zea mays), most protocols employ a paper towel roll method for experiments, which often involves wrapping maize seedlings in wet, sterile germination paper. What the paper towel roll method lacks, however, is the ability to image the roots over time without risk of contamination. Here, we describe a sterile plate growth assay that contains Murashige-Skoog medium to grow seedlings starting two days after germination. This protocol uses a section of a paper towel roll method to achieve uniform germination of maize seedlings, which are sterilely transferred onto large acrylic plates for the duration of the experiment. The media can undergo modification to include an assortment of plant hormones, exogenous sugars, and other chemicals. The acrylic plates allow researchers to freely image the plate without disturbing the seedlings and control the environment in which the seedlings are grown, such as modifications in temperature and light. Additionally, the protocol is widely adaptable for use with other cereal crops.
Key features
• Builds upon plate growth methods routinely used for Arabidopsis seedlings but that are inadequate for maize.
• Real-time photographic analysis of seedlings up to two weeks following germination.
• Allows for testing of various growth conditions involving an assortment of additives and/or modification of environmental conditions.
• Samples are able to be collected for genotype screening.
Graphical overview
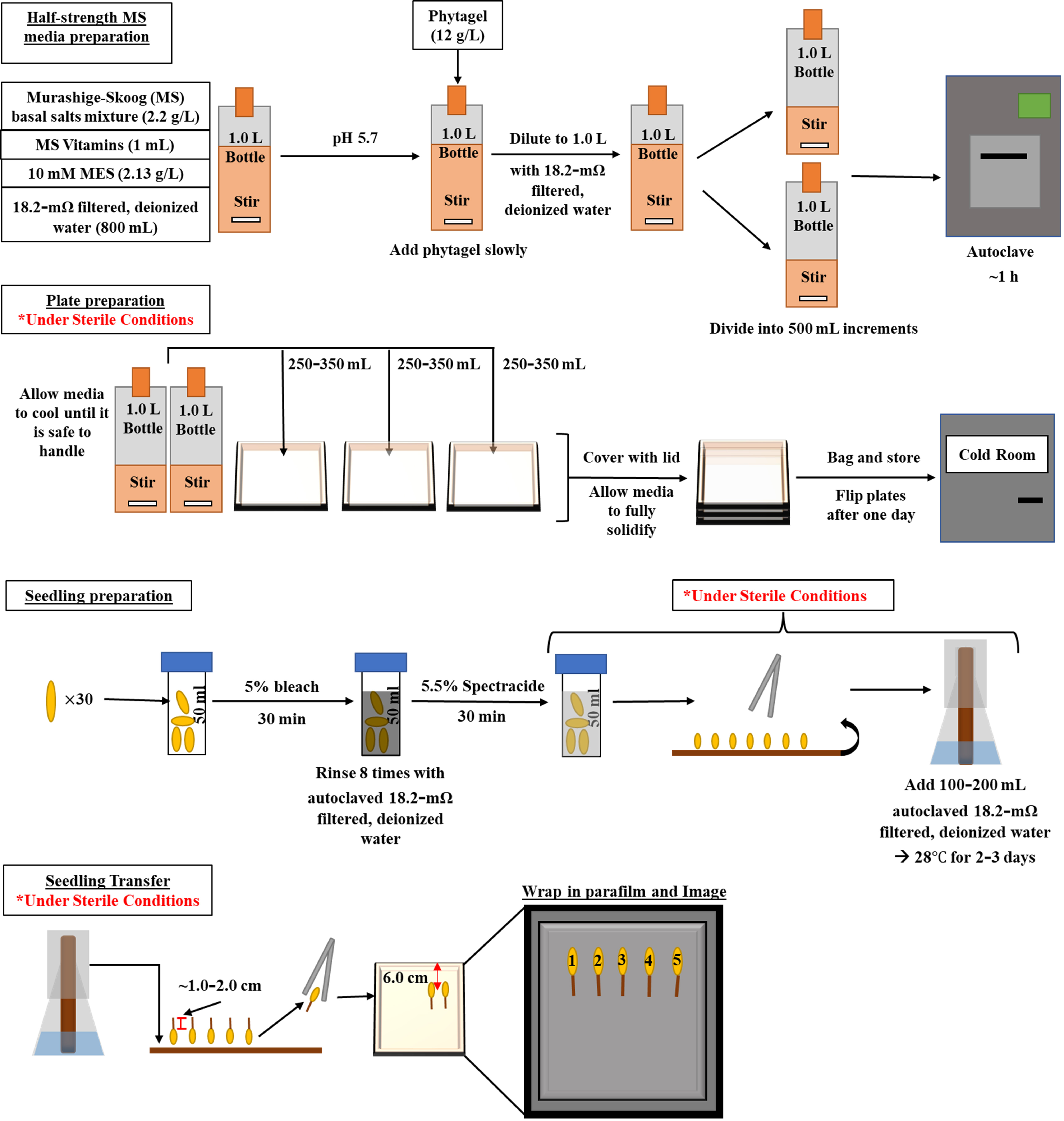
Background
Murashige-Skoog (MS) medium solutions have been used to provide the essential nutrients for plant development in root growth assays and can readily undergo alterations to incorporate a broad range of nutrients, chemicals, exogenous sugars, and plant hormones. A variety of these assays are routinely used in growing Arabidopsis thaliana (LaMontagne et al., 2016; Collins et al., 2020; Deslandes-Hérold et al., 2022; Mathew et al., 2023; Montpetit et al., 2023) and have included additives such as sodium chloride (Zhou et al., 2018), polyethylene glycol (PEG) (Zheng et al., 2016), and gibberellins (Unterholzner et al., 2015). For crops that produce larger seeds, such as maize, few protocols have been described employing MS media for seedling development. Most protocols utilize paper towel rolls to germinate and grow seedlings (Abdel-Ghani et al., 2016; Draves et al., 2022). Similarly, other additives can be incorporated into paper towel roll methods. A disadvantage to this method, however, includes imaging. To image the roots consistently, the rolls need to be removed from the incubator and laid out on a surface long enough to collect data before re-rolling and placing them back in the incubator, risking contamination if not carefully done. Imaging the progress of root growth over designated time intervals becomes restricted (Dowd et al., 2019).
Here, we introduce a sterile plate growth assay consisting of a half-strength MS medium used to grow maize seedlings starting two days after germination (DAG). This procedure is based on a protocol in a previously published paper (Julius et al., 2021) and was modified extensively to produce an assay functional to a wide range of experiments. Like paper towel roll methods, which can control environmental conditions such as temperature and light (Hochholdinger, 2009), this procedure also grants researchers control over the environment in which the seedlings develop. Moreover, the plates can be easily removed from an incubator and imaged at any time without disturbing the seedlings. The procedure can grow seedlings under a variety of conditions and treatments in a sterile environment, allowing researchers to investigate numerous effects in the embryonic root and shoot systems of maize and other cereal crops.
Materials and reagents
Materials
Three 24.5 cm × 24.5 cm acrylic square plates (Fischer Scientific, catalog number: 12-565-224)
Four 1.0 L bottles (w/caps)
Two stir bars
One spatula
One piece of 4" × 4" weigh paper (Fisherbrand, catalog number: 09-898-12B)
One piece of 6" × 6" weigh paper (Fisherbrand, catalog number: 09-898-12C)
One 1.0 L graduated cylinder
One 2-gallon zip-loc bag
One black marker
One 1.0 L Erlenmeyer flask
One 1.0 L beaker
One piece of 38# Regular weight seed germination paper, 10" × 15" (Anchor Paper Company, catalog number: SD3815L)
Three sets of flathead forceps
One 50 mL conical tube (Fisher Scientific, catalog number: 14-955-239)
Parafilm; 15 square (or 75 cm) strips
Aluminum foil
Plastic 6" ruler
Biological materials
This protocol was initially optimized to successfully grow maize seedlings of the B73 cultivar. Subsequent studies using other chemicals, maize mutants, and cereal seeds were performed (Slewinski and Braun, 2010; Tran et al., 2019; Huang et al., 2020; Babst et al., 2021), and examples will be provided later in this protocol. However, we recommend practicing this protocol with B73 seedlings.
Maize seedlings (B73 background)
Reagents
Bleach (liquid)
Ethanol
Spectracide (Immunox multi-purpose fungicide spray concentrate; any hardware store)
Murashige-Skoog basal medium (MP Biomedicals LLC, catalog number: 2623122)
MS vitamin solution (MP Biomedicals LLC, catalog number: 2625149)
MES (Millipore Sigma, catalog number: 6110-OP)
Phytagel (Sigma-Aldrich, catalog number: P8169)
Sodium hydroxide (NaOH) (Fisher Scientific, catalog number: S318-1)
Solutions
5% bleach (see Recipes)
5.5% spectracide (see Recipes)
70% ethanol (see Recipes)
Half-strength MS media (see Recipes)
Recipes
5% bleach
Note: Use 18.2 mΩ filtered, deionized water.
Reagent Final concentration Quantity (1.0 L) Bleach 5% 50 mL H2O n/a 950 mL Total n/a 1,000 mL 5.5% spectracide
Note: Use 18.2 mΩ filtered, deionized water.
Reagent Final concentration Quantity (1.0 L) Spectracide 5.5% 55 mL H2O n/a 945 mL Total n/a 1,000 mL 70% ethanol
Note: Use 18.2 mΩ filtered, deionized water. The solution will be mixed in a spray bottle; pour volumes as needed.
Reagent Final concentration Quantity (1.0 L) Ethanol (absolute) 70% 700 mL H2O n/a 300 mL Total n/a 1,000 mL Half-strength MS media
Note: Use 18.2 mΩ filtered, deionized water. Preparation of 1.0 L of media makes approximately three plates; see Procedure C for preparation instructions. Any additional ingredients must be calculated and experimented with independently.
Murashige-Skoog (MS) basal salts mixture (2.2 g/L)
MS vitamins at 1× (1 mL of vitamins per 1.0 L of media)
10 mM MES (2.13 g/L)
Phytagel (12 g/L)
18.2 mΩ filtered, deionized water (autoclaved)
Optional: Other additives can be incorporated, but the volumes need to be calculated for a 1.0 L solution.
Equipment
Incubator (Lindberg/Blue M, model: GI200C) set at 28 °C
Laminar flow hood
pH meter
200–1,000 pipettor
200–1,000 pipette tips (sterile aerosol barrier tips)
Orbital shaker (VWR, model: DS-500)
Stir plates
Weigh scale
Flatbed scanner (Ricoh, model: MP C6503)
Software
ImageJ (version 1.53k) (https://imagej.nih.gov/ij/)
SmartRoot (version 4.21) (https://smartroot.github.io/)
PDF to JPG converter (https://pdftoimage.com/pdf-to-jpg)
Smartphone app to convert images to PDFs, such as Microsoft Lens-PDF Scanner
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Roberts, J. T., McCubbin, T. J. and Braun, D. M. (2023). A Plate Growth Assay to Quantify Embryonic Root Development of Zea mays. Bio-protocol 13(20): e4858. DOI: 10.21769/BioProtoc.4858.
分类
植物科学 > 植物发育生物学 > 综合
植物科学 > 植物生理学 > 非生物胁迫
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













