- EN - English
- CN - 中文
Microscopy and Plate Reader–based Methods for Monitoring the Interaction of Platelets and Tumor Cells in vitro
基于显微镜和读板仪的体外监测血小板与肿瘤细胞相互作用的方法
(*contributed equally to this work) 发布: 2023年10月20日第13卷第20期 DOI: 10.21769/BioProtoc.4856 浏览次数: 2359
评审: Dipak Kumar PoriaJibin SadasivanEduardo ListikAnonymous reviewer(s)
Abstract
Platelets and their activation status play an essential role in cancer metastasis. Therefore, the anti-metastatic potential of antiplatelet drugs has been investigated for many years. However, the initial screening of these antiplatelet drugs to determine which agents can inhibit the interactions of platelets and tumor cells is very limited due to reliance upon expensive, time-consuming, and low-throughput animal experiments for screening. In vitro models of the platelet–tumor cell interaction can be a useful tool to rapidly screen multiple antiplatelet drugs and compare their ability to disrupt platelet–tumor cell interactions, while also identifying optimal concentrations to move forward for in vivo validation. Hence, we adopted methods used in platelet activation research to isolate and label platelets before mixing them with tumor cells (MDA-MB-231-RFP cells) in vitro in a static co-culture model. Platelets were isolated from other blood components by centrifugation, followed by fluorescent labeling using the dye CMFDA (CellTrackerTM Green). Labeling platelets allows microscopic observation of the introduced platelets with tumor cells grown in cell culture dishes. These methods have facilitated the study of platelet–tumor cell interactions in tissue culture. Here, we provide details of the methods we have used for platelet isolation from humans and mice and their staining for further interaction with tumor cells by microscopy and plate reader–based quantification. Moreover, we show the utility of this assay by demonstrating decreased platelet–tumor cell interactions in the presence of the T-Prostanoid receptor (TPr) inhibitor ifetroban. The methods described here will aid in the rapid discovery of antiplatelet agents, which have potential as anti-metastatic agents as well.
Key features
• Analysis of platelet–tumor cell binding dynamics.
• In vitro methods developed for measuring platelet–tumor cell binding to enable rapid testing of antiplatelet and other compounds.
• Complementary analysis of platelet–tumor cell binding by imaging and fluorimetry-based readings.
• Representative results screening the effect of the antiplatelet drug, ifetroban, on platelet–tumor cell binding using the protocol.
• Validation results were presented with both a TPr agonist and ifetroban (antagonist).
Graphical overview
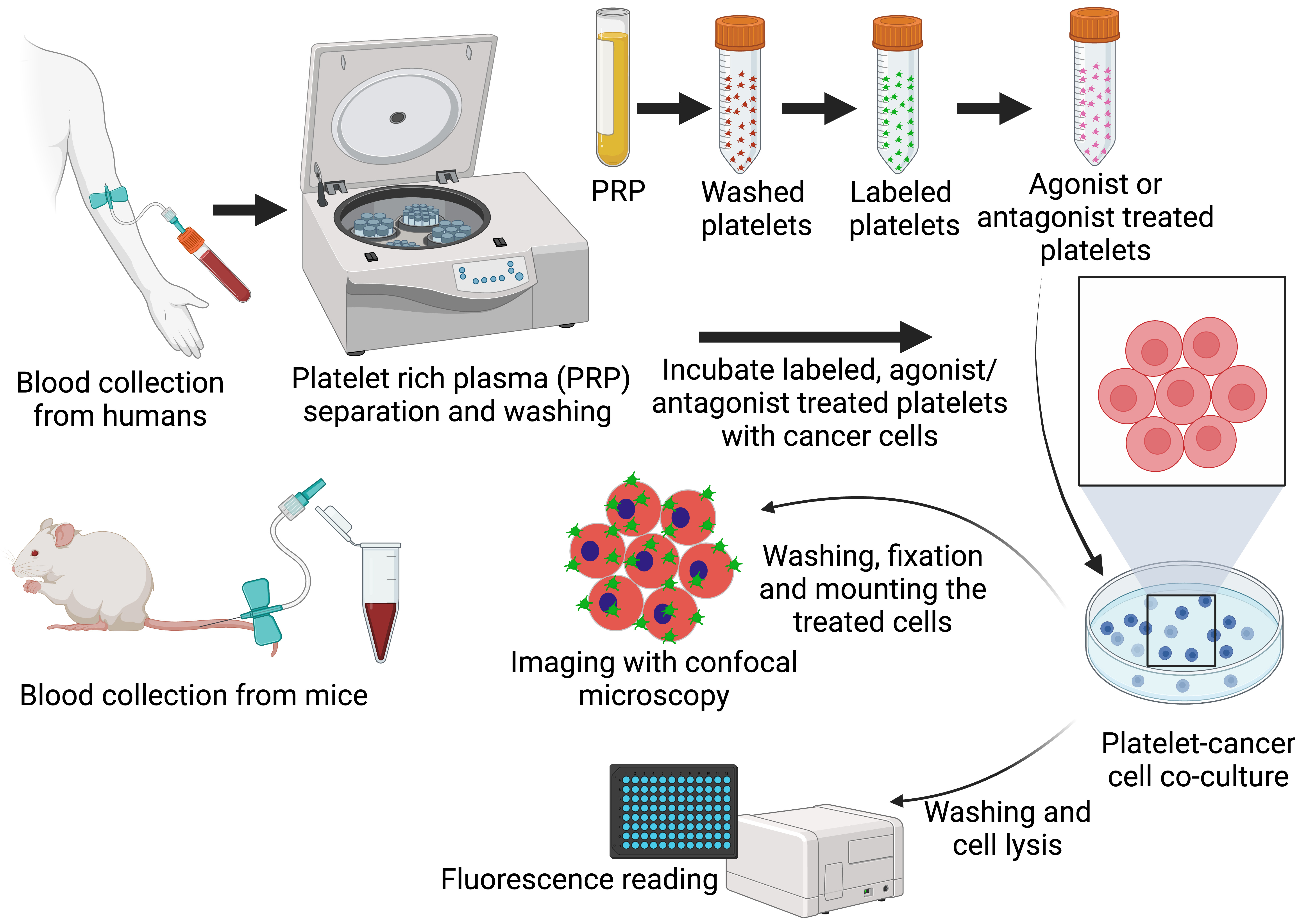
Representative overview of the process to isolate and label platelets, incubate platelets and tumor cells in the presence of antiplatelet agents, and image and/or quantify platelet–tumor cell interactions.
Background
Platelets are well known to play various crucial roles in hemostasis and thrombosis. However, growing evidence suggests that platelets are significantly involved in cancer metastasis as well (Gay and Felding-Habermann, 2011). Further, preclinical and clinical evidence has shown that platelets promote tumorigenesis and metastasis by paracrine interactions with cancer cells (Labelle et al., 2011). Cancer cells change platelet behavior by directly inducing tumor–platelet aggregates and triggering platelet activation (granule and extracellular vesicle release, altered phenotype, etc.). Further, platelet activation has been directly or indirectly linked to the dissemination of cancer cells, cancer cell survival within the circulation, and the extravasation of cancer cells at distant sites of metastasis (Xu et al., 2018). Since platelet activation pathways are likely contributors to cancer growth and metastasis, antiplatelet drugs have immense potential for the treatment of cancer metastasis by inhibiting a myriad of events that drive cancer growth and metastasis (Gay and Felding-Habermann, 2011; Wojtukiewicz et al., 2017; Lucotti et al., 2019; Tao et al., 2021). For instance, we recently discovered single nucleotide polymorphisms in TBXA2R, a gene expressed on platelets, that correlate significantly with metastasis of many solid cancers, including breast, melanoma, colon, and lung (Werfel et al., 2020). Further, we found that both hyperactivated and wild-type TPr (Thomboxane A2-Prostanoid receptor)—the protein encoded by TBXA2R—contribute to cancer metastasis in animal models. The safe and selective inhibitor of TPr, ifetroban, was able to reverse this effect and significantly reduce metastasis of cancer cell lines in the same animal models. However, antiplatelet drug efficacy against platelet–tumor cell interactions is challenging to study in vitro due to difficulties in fully replicating the dynamic and intricate processes that occur in vivo and often requires screening directly in rodent models of metastasis. These rodent studies are expensive, time-consuming, and low-throughput, which is particularly burdensome when a large number of antiplatelet drugs need to be screened. Hence, in order to rapidly screen for agents with anti-metastatic potential through disrupting platelet–tumor cell interactions, we have developed methods for the fluorescent labeling of platelets and the study of platelet–tumor cell interactions in vitro using Red Fluorescent Protein (RFP)-expressing breast cancer cells (RFP-MDA-MB-231). Using these methods, we have carried out studies to quantify the interaction of platelets and tumor cells in culture, alongside other reports also showing that platelets play key roles in the survival and spreading of tumor cells early during the metastatic cascade (Ponert et al., 2018; Lucotti et al., 2019).
Materials and reagents
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A3059)
Coulter counter (Beckman; 50 μM aperture tube, 3–30 fl particles)
CellTrackerTM Green CMFDA Dye (Thermo ScientificTM, catalog number: C7025)
Dextrose (Sigma-Aldrich, catalog number: D9434-250)
DPBS (Ca2+/Mg2+ free) (Gibco, catalog number: 14190359)
Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, catalog number: 11965118)
EDTA (Sigma-Aldrich, catalog number: E5134)
Fetal bovine serum (FBS) (Gibco, catalog number: 26140079)
Calcium-free Hank’s Balanced Salt Solution (HBSS) (Sigma-Aldrich, catalog number: H9394)
HEPES (Sigma-Aldrich, catalog number: H2034)
Hydrochloric acid (Thermo Fisher ScientificTM, catalog number: A466-2)
Ifetroban (Medchem express, catalog number: HY-105218)
Mycoplasma-free MDA-MB-231-RFP cells (Gentarget Inc, catalog number: SC057-Bsd), and other tumor cells/xenografts fluorescent protein–incorporated or later staining in the confocal step models can be tested in a similar way
Paraformaldehyde 16% aqueous solution EM grade (Electron Microscopy Sciences, catalog number: 15710-S)
PBS, pH 7.4 (Thermo Fisher ScientificTM, catalog number: 10010023)
Penicillin-streptomycin (P/S; 10,000 U/mL) (Gibco, catalog number: 15140122)
Potassium chloride (KCl) (Thermo Fisher ScientificTM, catalog number: P217-500)
Prostaglandin E1 (PGE1) (Thermo Fisher ScientificTM, catalog number: P5515)
Sodium citrate (Sigma-Aldrich, catalog number: C8532)
Sodium chloride (NaCl) (MP Biomedicals, catalog number: 194848)
Sodium hydrogen carbonate (NaHCO3) (Sigma-Aldrich, catalog number: S6297-250)
Sodium phosphate monobasic (NaH2PO4) (Sigma-Aldrich, catalog number: 71505-250)
TrypLETM Express (Gibco, catalog number: 12604-021) or 0.05% Trypsin/EDTA (Gibco, catalog number: 15400-054)
U46619 (Cayman Chemicals, catalog number:16450)
Vectashield Antifade Mounting Medium with DAPI (Cole-Parmer®, catalog number: H-1200-10)
Triton-X 100 (Thermo Fisher ScientificTM, catalog number: A16046)
Solutions
Sodium citrate for anticoagulation of blood (see Recipes)
HBSS with EDTA (see Recipes)
DMEM complete media (see Recipes)
Washing buffer for platelets (see Recipes)
Resuspension buffer for platelets (see Recipes)
MTH buffer (see Recipes)
Recipes
Sodium citrate for anticoagulation of blood
Prepare a 3.8% solution of sodium citrate by dissolving 380 mg of sodium citrate in 7 mL of water (ddH2O).
Adjust pH to 7 with concentrated hydrochloric acid.
Add water to a final volume of 10 mL.
Store the solution at 2–8 °C for six months. Allow the solution to reach room temperature before use.
To anticoagulate blood with 0.38% sodium citrate final concentration, add 1 mL of 3.8% sodium citrate solution to 9 mL of whole blood. Adjust the volume of sodium citrate according to the final blood volume.
HBSS with EDTA
Add 250 μL of 1 M EDTA to 50 mL of HBSS and mix well.
Adjust pH to 6.4 with concentrated hydrochloric acid.
Store the solution at 2–8 °C for two weeks. Allow the solution to reach room temperature before use.
DMEM complete media
Remove the 50 mL of plain media from the fresh DMEM bottle in the cell culture hood.
Add 50 mL of heat-inactivated FBS to the DMEM bottle containing 450 mL of plain media.
Add 5 mL of Pen/Strep reagent (100×) to the above mixture and mix it gently.
Washing buffer for platelets
Add 0.1% wt/vol NaHCO3 + 0.1% wt/vol BSA + 1 mM EDTA (Mw = 380.35 g/mol).
Add 10% MTH (Recipe 6) vol/vol in dH2O.
Prepare all of them in ultrapure distilled water.
Sterilize each buffer after preparation using a sterile filter unit inside the cell culture hood.
Resuspension buffer for platelets
Add 0.1% wt/vol NaHCO3 + 0.1% wt/vol BSA.
Add 10% MTH vol/vol in dH2O.
Prepare all of them in ultrapure distilled water.
Sterilize each buffer after preparation using a sterile filter unit inside the cell culture hood.
MTH buffer
Add 134 mM NaCl (Mw = 58.44) + 0.3 mM NaH2PO4·2H2O (Mw = 156.01).
3 mM KCl (Mw = 74.55) + 5 mM HEPES (Mw = 238.3) + 5 mM dextrose (Mw = 180.16) + 2 mM MgCl2 (Mw = 203.3)
Prepare all of them in ultrapure distilled water.
Sterilize each buffer after preparation using a sterile filter unit inside the cell culture hood.
Laboratory supplies
Pipette tips (Gilson, catalog numbers: F167104, F167103, F167101)
Pipettes (Eppendorf, catalog numbers: 3123000012, 3123000020, 3125000109, 3123000055, 3123000063)
1 mL syringe (BD, catalog number: 309628)
10 mL syringe (BD, catalog number: 305959)
50 mL syringe (BD, catalog number: 300865)
0.22 μm syringe filter (Millex®, catalog number: SLGP033RS)
21 G syringe needle (Sarstedt, catalog number: 85.1162)
23 G syringe needle (BD, catalog number: 300800)
27 G syringe needle (BD, catalog number: 302200)
1.5 mL microcentrifuge tubes (Thermo ScientificTM, catalog number: 69715)
2 mL microcentrifuge tubes (Thermo ScientificTM, catalog number: 69720)
15 mL centrifuge tubes (Thermo ScientificTM, catalog number: 339650)
Black 96-well plate (Thermo ScientificTM, catalog number: 229411)
100 mm cell culture dishes (Thermo ScientificTM, catalog number: 150464)
48-well cell culture plate (Thermo ScientificTM, catalog number: 8715064)
Collagen-coated 35 mm dish coverslip (MatTek Corporation, catalog number: NC9125998)
Cell counting chamber slides (Thermo ScientificTM, catalog number: C10314)
Transwell® inserts (Corning, catalog number: 3401)
Round-bottom 10 mL Plastic centrifuge tube (Thermo Fisher ScientificTM, catalog number: 02-689-6, BD VacutainerTM supplier)
EDTA/heparin-coated or Microhematocrit capillary tube (Thermo Fisher ScientificTM, catalog number: 22-362566)
Equipment
-20 °C and -80 °C freezers
Centrifuge with swing-out rotor suitable for 15 mL centrifugation tubes (Eppendorf, model: 5810R)
Liquid nitrogen dewar (Thermo Fisher ScientificTM, catalog number: CY509113)
37 °C incubator (Thermo Fisher ScientificTM, catalog number: FSGPD20)
Mouse restrainer (Braintree Scientific, model: SHORTI STD)
Analytical weighing scale (Mettler Toledo, ME104E and ME204E)
Laminar flow cabinet (Thermo ScientificTM 1300 Series Class II, catalog number: 1323TS)
CO2 cell culture incubator (Thermo Fisher ScientificTM, HERAcell VIOS 160i, catalog number: 51030403)
Benchtop centrifuge (Eppendorf, model: 5810R)
Improved Neubauer cell counting chamber (EMS, catalog number: 68052-16)
LSETM low-speed orbital shaker (CorningTM, catalog number: 6780FP)
Countess II automated cell counter (Invitrogen, Thermo Fisher ScientificTM, catalog number: AMQAX1000)
Brightfield microscope (Thermo Fisher ScientificTM, catalog number: LMI6PH1)
Microhematocrit capillary tubes (Thermo Fisher ScientificTM, catalog number: 22-362574)
Microplate reader (BioTek, model: Synergy H1). Fluorescence intensity details: Top, fluorescein 2.5 pM (0.25 fmol/well 384-well plate); bottom, fluorescein 4 pM (0.4 fmol/well 384-well plate); bandpass = 18 nm (excitation and emission), fluorescein 0.25 pM (0.025 fmol/well 384-well plate); light source: Xenon flash lamp, wavelength range: 230–999 nm, 1 nm increment, bandpass: 4 nm (230–285 nm), 8 nm (> 285 nm) and wavelength range: monochromators: 250–700 nm, detection system: PMT detectors
Confocal microscope (LEICA SP8X, Wetzlar, Germany):
Objectives: HC PL APO CS2 40×/1.10 water
Lasers: 405 Diode (on), 442 diode, WLL (on, 70.00%)
Channels: Green, Gray, Blue, and Red
Laser lines (nm): 405, 442, 491, 554, 472, 491, 667, 670
Optimal settings: Scan mode (xyz), Scan speed (400 Hz), Magnification (40), Immersion (water), Pinhole (77.2 μm) and Emission wavelength (580 nm)
Software
Gen5, 3.05 (BioTek Instrument)
Prism, 9.2.0 (GraphPad)
Confocal microscopy image analysis software (Leica application suite 3.7.6)
Photoshop 21.1 (Adobe)
ImageJ 13.0.6 (imagej.nih.gov/ij/download/)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Toragall, V., Hale, E. J., Hulugalla, K. R. and Werfel, T. A. (2023). Microscopy and Plate Reader–based Methods for Monitoring the Interaction of Platelets and Tumor Cells in vitro. Bio-protocol 13(20): e4856. DOI: 10.21769/BioProtoc.4856.
分类
癌症生物学 > 侵袭和转移 > 细胞生物学试验 > 细胞分离和培养
细胞生物学 > 细胞分离和培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











