- EN - English
- CN - 中文
In vitro Quality Assessments of Perfluorocarbon Nanoemulsions for Near-infrared Fluorescence Imaging of Inflammation in Preclinical Models
用于临床前模型中炎症近红外荧光成像的全氟化碳纳米乳剂的体外质量评估
发布: 2023年10月05日第13卷第19期 DOI: 10.21769/BioProtoc.4842 浏览次数: 2073
评审: Alessandro DidonnaAnonymous reviewer(s)

相关实验方案
NMR waterLOGSY应用于药物开发项目中蛋白质-配体相互作用的检测
Carole J. R. Bataille [...] Timothy D. W. Claridge
2020年07月05日 6836 阅读
Abstract
Tracking macrophages by non-invasive molecular imaging can provide useful insights into the immunobiology of inflammatory disorders in preclinical disease models. Perfluorocarbon nanoemulsions (PFC-NEs) have been well documented in their ability to be taken up by macrophages through phagocytosis and serve as 19F magnetic resonance imaging (MRI) tracers of inflammation in vivo and ex vivo. Incorporation of near-infrared fluorescent (NIRF) dyes in PFC-NEs can help monitor the spatiotemporal distribution of macrophages in vivo during inflammatory processes, using NIRF imaging as a complementary methodology to MRI. Here, we discuss in depth how both colloidal and fluorescence stabilities of the PFC-NEs are essential for successful and reliable macrophage tracking in vivo and for their detection in excised tissues ex vivo by NIRF imaging. Furthermore, PFC-NE quality assures NIRF imaging reproducibility and reliability across preclinical studies, providing insights into inflammation progression and therapeutic response. Previous studies focused on assessments of colloidal property changes in response to stress and during storage as a means of quality control. We recently focused on the joint evaluation of both colloidal and fluorescence properties and their relationship to NIRF imaging outcomes. In this protocol, we summarize the key assessments of the fluorescent dye–labeled nanoemulsions, which include long-term particle size distribution monitoring as the measure of colloidal stability and monitoring of the fluorescence signal. Due to its simplicity and reproducibility, our protocols are easy to adopt for researchers to assess the quality of PFC-NEs for in vivo NIRF imaging applications.
Keywords: Near-infrared fluorescence (NIRF) (近红外荧光 (NIRF))Background
Macrophages with their pleiotropic functions, ranging from protective to pathological (Ross et al., 2021), are important therapeutic and diagnostic targets (Ardura et al., 2019). Macrophages are the ultimate link between pain and inflammation. They are major drivers of pathological processes in most inflammatory diseases from rheumatoid arthritis, osteoarthritis, and inflammatory bowel disease (IBD) to several types of cancer. Imaging macrophages in vivo has the potential to open a wide variety of research and clinical opportunities, as this would allow a more accurate assessment of inflammatory conditions and therapeutic response. Perfluorocarbon (PFC) nanoemulsions (PFC-NEs) labeling macrophages in vivo for the purpose of 19F MRI detection of inflammation (Temme et al., 2012) and tracking immune cells (Ahrens and Bulte, 2013) has been extensively studied in a multitude of preclinical models of IBD (Kadayakkara et al., 2010 and 2012), neurological diseases (Zhong et al., 2015), arthritis (Balducci et al., 2012), and organ rejection (Hitchens et al., 2011).
The introduction of fluorescent dyes to PFC-NEs serves two purposes: 1) detecting PFC-NE-labeled cells in ex vivo tissue samples by fluorescence microscopy, and 2) providing a complimentary tracer for near-infrared fluorescence (NIRF) imaging in vivo and ex vivo. There are two distinct methods for fluorescent-dye incorporation into PFC-NEs. The first method requires chemical modification of dyes with fluorous tags (Lim et al., 2020) or their direct conjugation to the PFC oil (e.g., perfluoropolyether, PFPE) (Janjic et al., 2008; Patrick et al., 2013). The second method requires PFC-NEs formulated as “triphasic” NEs, which consist of PFC (fluorous phase) and hydrocarbon oil (synthetic or natural/organic phase) combined into an internal phase and then dispersed into water (aqueous), as the third phase (Janjic et al., 2014). These types of NEs allow for high and stable incorporation of one or more fluorescent dyes (Patel et al., 2013a and 2013b; Nichols et al., 2021).
Our lab has reported multiple examples of triphasic theranostic NEs used to encapsulate and deliver non-steroidal anti-inflammatory drugs (e.g., celecoxib) (Herneisey et al., 2019; Liu et al., 2020) and natural products (resveratrol, curcumin) (Herneisey et al., 2016; Herneisey and Janjic, 2023), rendering them multimodal (19F MR/NIRF) imaging and drug delivery agents or theranostics. When theranostic NEs are used in vivo, NIRF imaging tracks macrophages as they respond to the delivered drug by monitoring changes in their infiltration patterns at the site of inflammation caused by injury, surgery, or other insults. Specifically, we demonstrated that COX-2-inhibiting theranostic PFC-NEs have both anti-inflammatory and analgesic effects in vivo in rodent models of neuropathic (Janjic et al., 2018; Saleem et al., 2019) and inflammatory pain (Liu et al., 2020). NIRF imaging was used in these studies to measure macrophage infiltration changes due to the drug delivered. Ex vivo immunofluorescence confirms the theranostic PFC-NE associated with the intended target, where the fluorescent nanoemulsion is co-registered with the specific cell marker (Saleem et al., 2019; Liu et al., 2020). It is critical that PFC-NEs retain their droplet size distribution and fluorescence signal from the time of manufacture, during storage, and during use in animal studies. Therefore, it is important to regularly test the prepared formulations for both stability and any batch-to-batch variations. Any differences in fluorescence signal intensity between PFC-NE batches and in the presence of the drug could lead to ambiguous or inconclusive in vivo imaging results. We developed a series of tests that serve as quality control assessments for PFC-NEs as NIRF imaging agents. Figure 1 shows the conceptual framework for the presented protocols and how they are utilized in validating PFC-NEs ex vivo for the purpose of in vivo macrophage tracking in preclinical models, followed by ex vivo immunofluorescence in tissues of interest. Based on our prior work and by utilizing quality-by-design (QbD) methodologies, we established critical quality attributes (CQAs) that, when met, render PFC-NEs effective in vitro and in vivo as NIRF imaging agents for macrophage tracking (Herneisey et al., 2019; Saleem et al., 2019; Liu et al., 2020; Nichols et al., 2021; Herneisey and Janjic, 2023). In the current protocol, we describe stress tests and associated instrumental measurements that can be easily performed to obtain reliable results on colloidal and fluorescence stability of PFC-NEs under varied conditions: filtration, storage at physiological temperatures, and centrifugation. These conditions model the types of stressors the PFC-NEs are subjected to during their use as either ex vivo cell-labeling agents or in vivo drug delivery and imaging agents. Observed changes in PFC-NE size distribution and fluorescence during the stress tests must fall within the CQA specifications for the formulation to be effective for in vitro and in vivo biological testing as a NIRF imaging agent. In this protocol, we provide a list of quality tests and describe their methodology and associated CQAs for NIRF-labeled PFC-NEs.
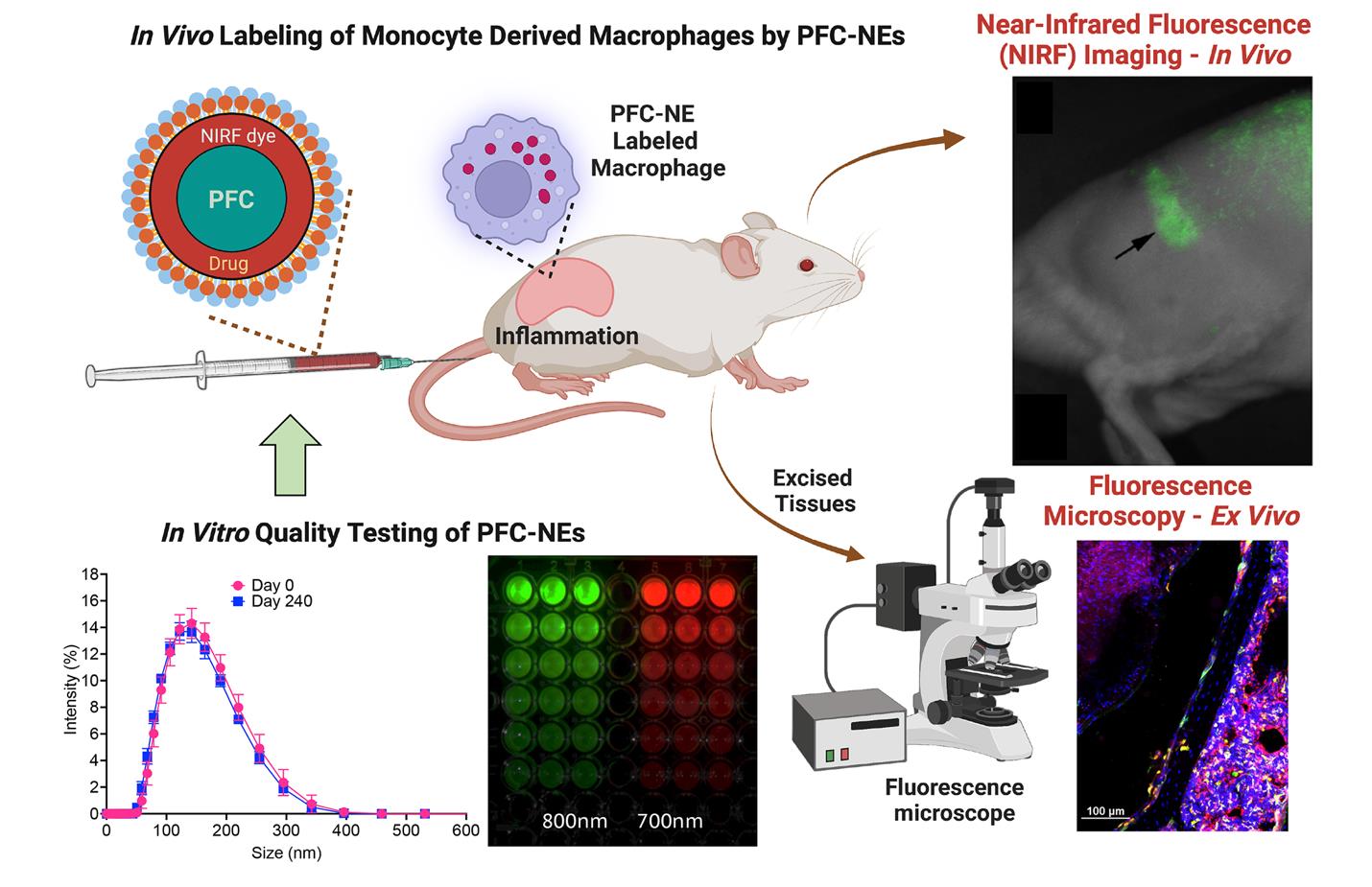
Figure 1. Role of quality assessments of fluorescently labeled perfluorocarbon nanoemulsions (PFC-NEs) in preclinical imaging of inflammation in rodent models. The PFC-NEs are tested for size distribution, colloidal and fluorescence stability (lower left panels), and subjected to cell culture testing to measure macrophage uptake and viability (data not shown). Following PFC-NEs tail vein injection in rodents, monocyte-derived macrophages uptake the PFC-NEs and carry them to the site of inflammation. As PFC-NE-labeled macrophages accumulate in the region of inflammation, the near-infrared fluorescent (NIRF) signal also increases. This can be monitored in real time using preclinical imagers, either in vivo or ex vivo. After the experiment, excised tissues can be examined using fluorescence microscopy for the presence of PFC-NE-labeled macrophages. NIRF rat image (top right) reproduced from (Vasudeva et al., 2014); macrophages’ immunofluorescence image from diabetic mice (bottom right) reproduced from (Nichols et al., 2021). Both images are reproduced under the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by-nc/4.0/).
Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM) (Corning, catalog number: 10-013-CV), store at 4 °C
Fetal bovine serum (FBS) (ATCC, catalog number: 30-2020TM), aliquot and store at -20 °C
Freshly prepared, 100–200 nm sized nanoemulsions (please check references for the method of preparation of nanoemulsions)
24-well plates (Corning, VWR, catalog number: 10062-896)
96-well plates (Corning, VWR, catalog number: 29442-056)
Microcentrifuge tubes (Fisherbrand Premium Microcentrifuge Tubes, catalog number: 05-408-129)
MilliporeSigmaTM MillexTM-GS Sterile Syringe Filter Unit, MCE, 0.22 and 0.45 μm (catalog numbers: SLGSR33SB and SLHA033SS)
FisherbrandTM disposable cuvettes (Fisher Scientific, catalog number: 14-955-127)
Parafilm (Sigma-Aldrich, P7793)
Type I ultrapure HPLC grade water (18.2 MΩ·cm) generated using PURELAB® Option-Q system
DiR [1,1’-Dioctadecyl-3,3,3’,3’-Tetramethylindotricarbocyanine Iodide, DiIC18(7)] (Thermo Scientific, catalog number: D12731)
DiD [DiIC18(5); 1,1’-dioctadecyl-3,3,3’,3’- tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt] (Thermo Scientific, catalog number: D7757)
Fluid thioglycolate medium (Millipore, catalog number: STBMCTM12)
Trypticase soy broth (Millipore, catalog number: STBMTSB12)
Equipment
Odyssey Classic Imager (Li-COR Inc., Lincoln, NE)
Odyssey M Imaging System (Li-COR Inc., Lincoln, NE)
Pearl Small Animal Imaging System (Li-COR Inc., Lincoln, NE)
37 °C incubator (Fisher Scientific, IsoTemp® 500 Series)
Zetasizer NanoZS (Malvern, UK)
Prism R Refrigerated Microcentrifuge (Labnet International Inc.)
Software and datasets
Image Studio Ver 5.2 (Li-COR Biosciences, U.S.)
Zetasizer Software 7.13 (Malvern Panalytical)
GraphPad Prism, 10.0.0
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Janjic, J. M., McCallin, R., Liu, L., Crelli, C., Das, A. C. and Troidle, A. (2023). In vitro Quality Assessments of Perfluorocarbon Nanoemulsions for Near-infrared Fluorescence Imaging of Inflammation in Preclinical Models. Bio-protocol 13(19): e4842. DOI: 10.21769/BioProtoc.4842.
分类
医学 > 发炎
生物物理学 > 红外光谱
生物物理学 > 核磁共振波谱法 > NMR成像技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link