- EN - English
- CN - 中文
Human Schwann Cells in vitro III. Analytical Methods and a Practical Approach for Quality Control
体外人雪旺细胞 III. 分析方法和质量控制的实用方法
发布: 2023年11月20日第13卷第22期 DOI: 10.21769/BioProtoc.4840 浏览次数: 2640
评审: Vivien J. Coulson-ThomasDhruv Rajanikant PatelWeiyan Jia
Abstract
This paper introduces simple analytical methods and bioassays to promptly assess the identity and function of in vitro cultured human Schwann cells (hSCs). A systematic approach is proposed to unequivocally discriminate hSCs from other glial cells, non-glial cells, and non-human SCs (authentication), identify hSCs at different stages of differentiation, and determine whether individual hSCs are proliferative or senescent. Examples of how to use distinct cell-based approaches for quality control and routine troubleshooting are provided to confirm the constitution (identity, purity, and heterogeneity) and potency (bioactivity) of hSC cultures from multiple sources. The bioassays are valuable for rapidly gauging the responses of hSCs to mitogenic and differentiating factors and ascertaining the cells’ basic properties before performing co-culture or cell grafting studies. The assays are image based and use adherent hSCs established in monoculture to simplify the experimental setup and interpretation of results. Finally, all sections contain thorough background information, notes, and references to facilitate decision making, data interpretation, and ad hoc method development for diverse applications.
Keywords: Bioassays (生物测定)Background
Human Schwann cell (hSC) cultures are valuable in vitro models for interrogating the biology of SCs during nerve development, maturation, and regeneration in normal and disease states. These cultures are also valuable as transplantable biologics to treat the injured or dysmyelinated central (CNS) and peripheral (PNS) nervous systems [reviewed in Guest et al. (2013); Monje et al. (2021c); Vallejo et al. (2022)]. Quality control testing of the cultured hSCs is needed for numerous reasons. First, hSC cultures are seldom pure and contain other proliferative cells from the endoneurium and the connective tissue layers. Second, the cultured hSCs differ from those in the original tissue as mature hSCs rapidly dedifferentiate after isolation and adapt to the in vitro environment. Third, non-cellular impurities and factors introduced during culture, such as animal serum and coating reagents, may alter hSC function. Altogether, these issues emphasize the importance of implementing robust analytical methods to promptly (1) confirm the hSC phenotype and the level of purity of the cell cultures; (2) evaluate the state of differentiation and biological activity of the hSCs; and (3) recognize and quantify myelin debris and other visible and subvisible impurities. Selecting suitable methodologies to address these elements will depend on the intended application. Cultured cells are manipulated biologics regardless of the manufacturing method used and need to be scrutinized thoroughly. Contrary to research-grade hSC cultures, those used clinically must meet the highest possible quality standards for identity and function (Bunge et al., 2017; Khan et al., 2021).
The goal of this paper is to describe easy-to-run analytical methods to reveal the purity, bioactivity, and often-changing constitution of donor-relevant hSC cultures by proposing the following: (1) to effectively identity hSCs using live and fixed cultures and discriminate them from cellular and non-cellular contaminants such as myelin, fibroblasts, non-human cells, and glial cells of CNS origin; and (2) to determine hSC function by means of proliferation, differentiation, and senescence assays (Figure 1). As explained in Protocol 1, immunological methods are recommended to reveal the phenotype and level of purity of the hSC cultures. Once the composition of the cultures is confirmed and the cells are authenticated for the species and tissue of origin (Protocol 2), investigators are encouraged to further interrogate their products using specific bioassays designed to evaluate the cellular responses to agonists known to exert an action on SC proliferation and differentiation (Protocol 3). Each protocol contains background information and notes to guide technical or logistical decisions regarding the analysis and interpretation of results. The troubleshooting section defines issues that researchers may encounter, along with appropriate approaches for problem resolution. Assessing cell viability, metabolic activity, microbial contamination, and cell transformation is important, but assays are not described here because they can be found elsewhere. Regarding contamination with cancerous cells, it should be noted that transformation of the hSCs in culture, or amplification of tumor cells imported from the tissue of origin, are unlikely events in hSC cultures from normal tissues (Bastidas et al., 2017).
The experimental conditions described in the following sections were optimized for adult nerve-derived hSC monocultures (adherent cells) obtained by traditional methods, irrespective of the source, passage, and stage of differentiation of the cells. However, our approach may be equally suitable for the analysis of other types of hSC cultures if enough cells (1 million or more) are available for the initial seeding. The use of multi-well plates is recommended for direct image analysis by fluorescence or light microscopy to reduce time, labor, and cost during experimentation and facilitate data interpretation. However, it is advisable to implement a systematic investigative approach (explained above) including more than one measure (or assay) to confirm the results. The suggested strategy is not intended to replace longer term in vitro (e.g., traditional co-culture systems) or in vivo studies (e.g., cell grafting). Some complex cellular responses requiring the interactions of hSCs with other cell types and the physical environment cannot be recapitulated under the simplified conditions of these experiments. Notwithstanding, the assays described here can be used as screening or discovery platforms to tackle various questions, since no consensus has been reached on how to approach quality control measures for SCs in culture. Future directions include scaling up the assays for a larger number of samples and developing quantitative approaches for specific readouts (e.g., myelin gene expression) relevant to nerve regeneration, therapy, or disease.
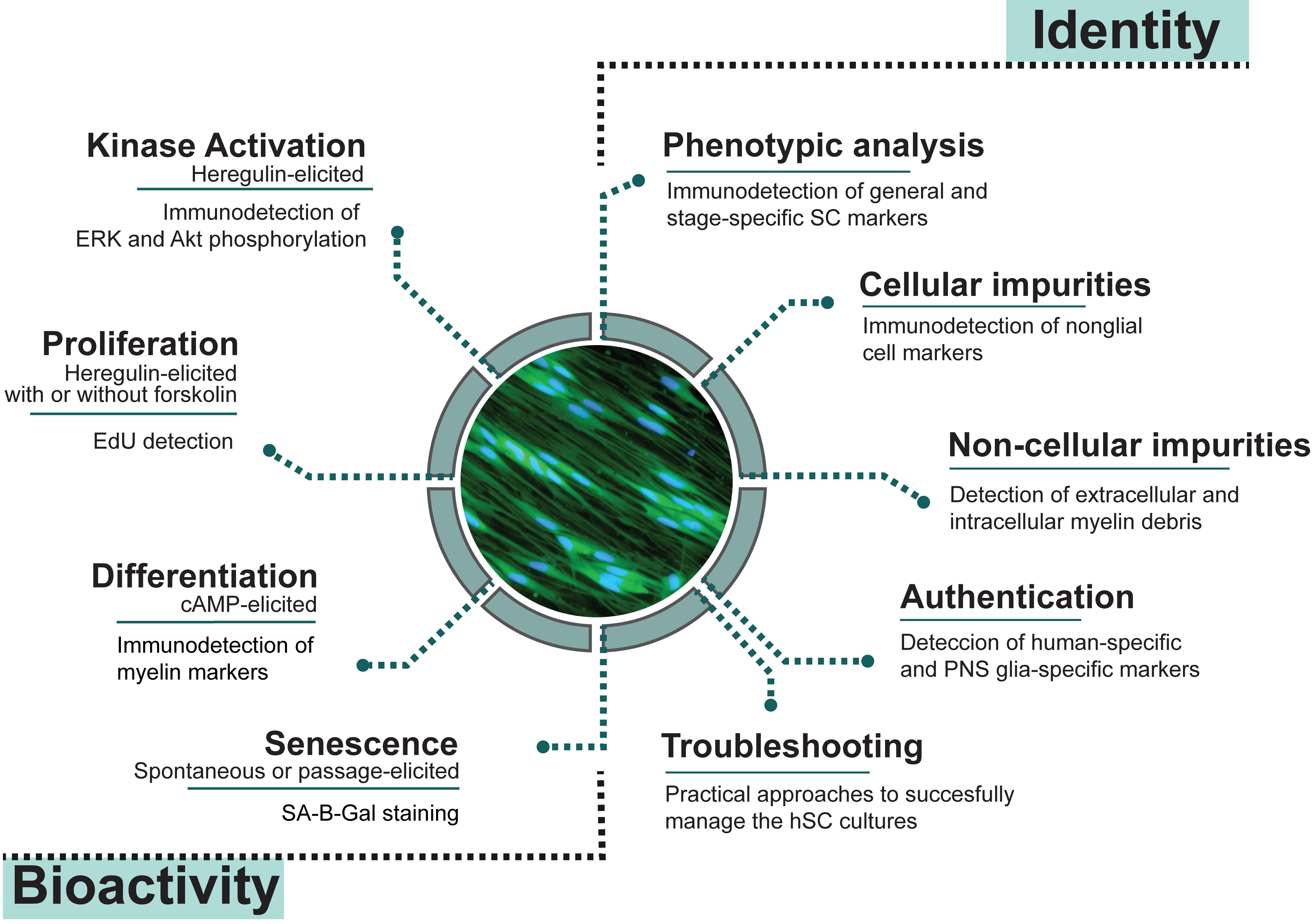
Figure 1. Quality control assessments for cultured human Schwann cells (hSCs). Measures of identity and bioactivity described in this study were selected based on their cell type specificity and reliability for scrutinizing hSC cultures in basic and translational research applications. Image: mitogen- and serum-starved hSCs stained with CellTrackerTM Green, a vital fluorescent dye.
Materials and reagents
Our cell culture protocols use research-grade materials, reagents, and solutions that are endotoxin-free and suitable for cell culture. Still, researchers are encouraged to assess the quality of relevant materials by appropriate methods to identify potentially toxic or incompatible elements in the culture medium, coating reagents, and plasticware.
All assays can be implemented using basic cell culture equipment and labware typically available in research labs. The description of products provided below is for reference only. Researchers may find comparable items from other providers. An exception is the primary antibodies (Table 1) because they have been validated carefully in our lab using cultured hSCs and nerve tissues. If other antibodies are selected, take into consideration that the ones used for rodent SC research may fail to recognize the respective antigens from humans. For this reason, it is important to confirm the specificity and reactivity of all antibodies in-house using proper positive and negative controls. Some of the suggested monoclonal antibodies were produced in our laboratory from hybridoma cell cultures but alternative ones are available from commercial sources (see Table 1). Lastly, the list below is not fully comprehensive. Additional technical details can be found in the accompanying papers (Aparicio and Monje, 2023; Monje, 2023).
Table 1. Useful antibodies to characterize human Schwann cells (hSCs) and non-glial cells established in cell culture. These antibodies were validated using traditional adult nerve-derived hSC monocultures. Staining with NGFR, Sox10, and S100B antibodies, alone or together with fibronectin, SMA (α-smooth muscle actin), and FAP (fibroblast activation protein/seprase) antibodies, is recommended for the initial characterization of hSCs and non-glial cells. We have not found a ubiquitous non-glial cell marker. Protocol 1C is suitable for staining with all antibodies except for anti-O1 and anti-O4, which can be accomplished only in live cells (Protocol 1B). A 1:200–1:500 starting dilution is suitable for most antibodies; the optimal concentration should be determined by the end user in the target cells. (*) Indicates that the antibodies are produced from hybridoma cell lines. MBP: myelin basic protein; MAG: myelin-associated glycoprotein; MPZ: myelin protein zero; GFAP: glial fibrillary acidic protein.
| Name of marker & subcellular localization | Product information | Expected results |
NGFR | • Mouse monoclonal 8737-IgG. ATCC # HB8737* | • Highly specific hSC marker. Expressed at high, homogeneous levels in all cultured hSCs. |
Sox10 | • Rabbit monoclonal. Abcam catalog number: ab155279. | • Highly specific hSC marker. Expressed at high, homogeneous levels in all hSCs. |
S100β | • Rabbit polyclonal. DAKO, catalog number: Z0311. • Mouse monoclonal. Sigma, catalog number: S2657 | • Highly specific hSC marker. High, homogeneous levels in all hSCs with occasional nuclear localization. |
Sox2 | • Rabbit polyclonal. Santa Cruz, catalog number: sc-20088. | • Highly specific hSC marker, heterogenously expressed in individual hSCs and reduced by CPT-cAMP stimulation. |
Nestin | • Mouse monoclonal. EMD Millipore, catalog number: MAB353. | • Highly specific hSC marker. Heterogeneously expressed (donor- or batch-dependent) and reduced by CPT-cAMP stimulation. |
GFAP | • Rabbit polyclonal. DAKO, catalog number: Z0334. | • Specific hSC marker expressed at low levels in expanded hSCs. |
MAG | • Mouse monoclonal. Chemicon, catalog number: MAB1567. | • Mostly in myelin debris. Expressed at low or undetectable levels in expanded hSCs. Induced with CPT-cAMP. |
MBP | • Rat monoclonal. MAB386 (Millipore, former Chemicon) | • Mostly in myelin debris. Non-myelin-associated MBP is rarely detectable in expanded hSC cultures. |
MPZ/P0 | • Chicken polyclonal. AB9352 (Millipore, former Chemicon) | • Mostly in myelin debris. Non-myelin-associated MPZ is rarely detectable in expanded hSC cultures. |
Erg2/Krox20 | • Rabbit polyclonal (non-commercial) | • Expressed at low levels in hSCs. Enhanced with CPT-cAMP. |
O4 | • Mouse monoclonal. O4-IgM * | • Expressed in all hSCs right after isolation but only in a proportion of the expanded hSCs. Enhanced with CPT-cAMP. |
O1 | • Mouse monoclonal. O1-IgM* | • Expressed in hSCs right after isolation. Expanded hSCs are O1- regardless of cAMP levels. |
Vimentin | • Rabbit monoclonal. Cell Signaling, catalog number: D21H3, 5741. | • Equally expressed in hSCs and fibroblasts at high, homogeneous levels. |
CD44 | • Mouse monoclonal. Cell Signaling, catalog number: 156-3C11. | • Equally expressed in hSCs and fibroblasts at high, homogeneous levels. |
Fibronectin | • Mouse monoclonal. Santa Cruz, catalog number: sc-8422. | • Filamentous or punctuated staining with significantly higher levels in fibroblasts as compared to hSCs. |
SMA | • Mouse monoclonal. Thermo Fisher: catalog number: MS113-PO. | • Expressed at high levels in a proportion of non-glial cells, possibly pericytes. hSCs display low levels of SMA. |
FAP | • Rabbit Monoclonal. Cell Signaling, catalog number: 66562. | • Expressed at high levels in a proportion of non-glial cells. hSCs do not typically express FAP. |
Thy1/CD90 | • Rabbit monoclonal. Abcam. Catalog number: 92574 Abcam | • Expressed at variable levels in a proportion of non-glial cells. Hard to detect by simple immunostaining. hSCs do not typically express Thy1. |
Supplies and consumables
Polypropylene conical-bottom centrifuge tubes, 15 and 50 mL (Corning, catalog numbers: 430791 and 430290)
Serological pipettes, 5, 10, and 25 mL, polystyrene, sterile (VWR)
Pasteur pipettes, polystyrene, individually wrapped for liquid disposal (VWR, Argos Technology, catalog number: 10122-560)
Laminin-coated cell culture dishes for cell expansion. 100 mm × 20 mm plates, polystyrene (Corning, catalog number: 353003) coated with a laminin substrate, as described in Andersen and Monje (2018)
Laminin-coated multi-well plates for analytical assays. Cell culture–treated 24-well plates, flat bottom, polystyrene (Corning, catalog number: 3524) coated sequentially with PLL and laminin, as described in (Andersen and Monje, 2018). (Optional) Use commercially available 24-well plates coated with poly-L-ornitine (PO) and laminin (BD Biosciences, catalog number 354659). Do not plate hSCs on uncoated surfaces. Polystyrene plates or chamber slides are preferred. Coverslips are not suitable since the hSCs are unstable and display an abnormal morphology on any glass surface
Paraformaldehyde (PFA) 20% stock solution (Electron Microscopy Sciences, catalog number: 15713)
Methanol (Sigma, catalog number: 154903) maintained at -20 °C for cell permeabilization. (Optional) 0.1% (v/v) Triton X-100 (Sigma, catalog number: 11332481001) prepared in D-PBS and stored at 4 °C
Laboratory wrapping film (Parafilm, catalog number: PM-996) and aluminum foil
Media, supplements, and other cell culture products
Distilled water, cell culture grade (Fisher Scientific, Gibco, catalog number: 15-230-147)
Dulbecco’s phosphate-buffered saline (DPBS) with calcium and magnesium, pH 7.2 (Thermo Fisher Scientific, Gibco, catalog number: 14190)
Hank’s balanced salt solution (HBSS) formulated without calcium or magnesium and containing phenol red, pH 7.2 (Thermo Fisher Scientific, Gibco, catalog number: 14170-112)
Dulbecco’s modified Eagle’s medium (DMEM) with high glucose and phenol red, pH 7.2 (Thermo Fisher Scientific, Gibco, catalog number: 11965092)
DMEM, Nutrient Mixture F-12 (DMEM/F-12), no glutamine, with phenol red (Thermo Fisher Scientific, Gibco, catalog number: 21331020)
De-complemented fetal bovine serum (FBS) (HyClone, catalog number: SV 30014.03), stored in aliquots at -80 °C
100× GlutaMAX supplement (Thermo Fisher Scientific, Gibco, catalog number: 35050061)
Gentamycin 50 mg/mL, 1,000× stock solution (Thermo Fisher Scientific, Gibco, catalog number: 15750-060)
HEPES buffer solution 1 M (Thermo Fisher Scientific, Gibco, catalog number: 15630-080)
Normal goat serum (GeneTex, catalog number: GTX73206), stored in aliquots at -80 °C
Forskolin (Sigma-Aldrich, catalog number: F68861); for a detailed protocol on preparation, storage, and use of forskolin stock solution, see Andersen and Monje (2018)
Heregulin-β1 (referred to as heregulin), HRG1-B1177-244 recombinant peptide (Preprotech, catalog number: G-100-03); for a detailed protocol on preparation, storage, and use of heregulin stock solution, see Andersen and Monje (2018)
CPT-cAMP stock solution (5 mM in DMEM), prepared from adenosine 3′,5′-cyclic monophosphate, 8-(4-chlorophenylthio), sodium salt (Calbiochem, catalog number: 116812); for a detailed protocol on preparation, storage, and use of CPT-cAMP stock solution, see Monje (2018)
Low proliferation medium (LP) (see Recipes)
High proliferation medium (HP) (see Recipes)
Starvation or D1 medium (DMEM/F12 - 1% FBS) (see Recipes)
PFA-based fixation solution (see Recipes)
Blocking solution (see Recipes)
Antibodies, dyes, and commercially available detection kits
Mouse monoclonal antibodies from hybridoma cell lines. Anti-nerve growth factor receptor (NGFR), anti-O4, and anti-O1 (Sommer and Schachner, 1981) in the form of conditioned medium produced from HB-8737 cells (also known as 200-3-G6-4, obtained from the American Type Culture Collection, ATCC), O4 cells, and O1 cells (kindly provided by Dr. Melitta Schachner), respectively. Researchers can refer to our publication (Ravelo et al., 2018) for technical details on our hybridoma culture protocols. Briefly, transfer the cell content of a hybridoma stock (1 × 106–2 × 106 cells/cryovial) directly into a T-75 flask containing Iscove’s modified Dulbecco’s medium (with phenol red) supplemented with 10% FBS and antibiotics. Culture the cells in suspension inside a CO2 incubator until the cultures are sufficiently dense and the medium becomes slightly acidic. Next, separate the culture supernatant from the cellular content by centrifugation to obtain conditioned medium enriched in monoclonal antibodies. The conditioned medium is often used without dilution, but the specificity and reactivity of each batch should be tested using appropriate positive control cells or tissues
Primary antibodies. pERK1/2/MAPK mouse monoclonal antibody (Santa Cruz, catalog number: sc7383); pAkt-Ser-473 rabbit polyclonal antibody (Santa Cruz, catalog number: sc7985); Akt rabbit polyclonal (Cell Signaling, catalog number: 9272); ERK2/MAPK rabbit polyclonal (Santa Cruz, catalog number: sc154); human nuclei (HNA), mouse monoclonal (Sigma, MAB1281, clone 235-1) or HNA mouse monoclonal (Abcam, ab191181). Other antibodies are listed in Table 1
Fluorescent secondary antibodies of the appropriate species and class, Alexa FluorTM-conjugated (Molecular Probes). Fluorochromes should be chosen and combined as optimal for visualization
FM4-64FX, fixable membrane stain (Invitrogen, catalog number: F34653). (Optional) FluoroMyelinTM (red or green) myelin stain (Invitrogen, catalog number: F34652)
Hoeschst-34580 (Molecular probes, catalog number: H21486). (Optional) 4′,6-Diamidino-2-Phenylindole, Dilactate, (DAPI, Invitrogen, catalog number: D3571)
Click-iT EdU (5-ethynyl-2′-deoxyuridine) Alexa-Fluor-594 Imaging Kit (Life Technologies, catalog number: C10339)
Senescence-associated (SA) β-Galactosidase (SA-β-Gal) staining kit (Cell Signaling Tech, catalog number: 9860)
VectaShieldTM antifade liquid mounting reagent for fluorescence (Vector Laboratories, catalog number: H-1000). (Optional) Prepare a homemade mounting reagent, as suggested in Ravelo et al. (2018), to control photobleaching and preserve the stained cultures. Add a sufficient volume of mounting reagent to fully cover the fixed cells (300 μL/well in a 24-well plate) or a couple of drops if a glass coverslip is mounted on top
Equipment
Biological safety cabinet, BL2 level (Thermo Scientific 1300 series class II, 1300 series, type A2)
Cell incubator set at 37 °C and 8%–9% CO2 (Thermo Scientific Forma, series II, water-jacketed)
Inverted phase contrast microscope (VWR) equipped with 10× to 40× phase contrast objectives and attached digital camera (VWR, V5MP)
Benchtop refrigerated centrifuge (Beckman CoulterTM, Allegra X-12R) equipped with swing bucket rotor (SX4750) and adapters for 50 and 15 mL tubes
Automated cell counter for the image-based counting of cells in suspension (Bio-Rad, TC20) or a hemocytometer for manual cell counting
Inverted fluorescence microscope (Olympus, IX71) for brightfield, phase contrast, and fluorescence microscopy; equipped with standard UV, FITC, and TRITC filter sets and attached digital camera
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Monje, P. V. (2023). Human Schwann Cells in vitro III. Analytical Methods and a Practical Approach for Quality Control. Bio-protocol 13(22): e4840. DOI: 10.21769/BioProtoc.4840.
分类
神经科学 > 周围神经系统 > 施万细胞
细胞生物学 > 基于细胞的分析方法
细胞生物学 > 细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link