- EN - English
- CN - 中文
GutMap: A New Interface for Analysing Regional Motility Patterns in ex vivo Mouse Gastrointestinal Preparations
GutMap:用于分析离体小鼠胃肠道制剂中区域运动模式的新界面
(*contributed equally to this work) 发布: 2023年10月05日第13卷第19期 DOI: 10.21769/BioProtoc.4831 浏览次数: 1601
评审: Durai SellegounderBruno MazetAnonymous reviewer(s)
Abstract
Different regions of the gastrointestinal tract have specific functions and thus distinct motility patterns. Motility is primarily regulated by the enteric nervous system (ENS), an intrinsic network of neurons located within the gut wall. Under physiological conditions, the ENS is influenced by the central nervous system (CNS). However, by using ex vivo organ bath experiments, ENS regulation of gut motility can also be studied in the absence of CNS influences. The current technique enables the characterisation of small intestinal, caecal, and colonic motility patterns using an ex vivo organ bath and video imaging protocol. This approach is combined with the novel edge detection script GutMap, available in MATLAB, that functions across Windows and Mac platforms. Dissected intestinal segments are cannulated in an organ bath containing physiological saline with a camera mounted overhead. Video recordings of gut contractions are then converted to spatiotemporal heatmaps and analysed using the GutMap software interface. Using data analysed from the heatmaps, parameters of contractile patterns (including contraction propagation frequency and velocity as well as gut diameter) at baseline and in the presence of drugs/treatments/genetic mutations can be compared. Here, we studied motility patterns of female mice at baseline and in the presence of a nitric oxide synthase inhibitor (Nω-Nitro-L-arginine; NOLA) (nitric oxide being the main inhibitory neurotransmitter of gut motility) to showcase the application of GutMap. This technique is suitable for application to a broad range of animal models of clinical disorders to understand underlying biological pathways contributing to gastrointestinal dysfunction.
Key features
• Enhanced video imaging analysis of gut contractility in rodents using a novel software interface.
• New edge detection algorithm to accurately contour curvatures of the gastrointestinal tract.
• Allows for output of high-resolution spatiotemporal heatmaps across Windows and Mac platforms.
• Edge detection and analysis method makes motility measurements accessible in different gut regions including the caecum and stomach.
Graphical overview
Background
Gut motility is critical for digestive function, including fermentation and mixing, stool formation, and microbial composition. This contractile activity is predominantly regulated by the intrinsic network of neurons within the gut, the enteric nervous system (ENS) (Furness, 2012). Motility patterns vary in the different regions of the gastrointestinal (GI) tract. In the small intestine, contractile activity serves to maximise exposure to digestive enzymes and absorption of nutrients, whereas the colon is responsible for the formation and expulsion of faeces and absorption of water and electrolytes. Several studies have reported characteristics of contractile patterning in the small and large intestine of small animal species, e.g., rat, mouse, and guinea pig (Lyster et al., 1995; Tonini et al., 1996; Costa et al., 2013 and 2015; Swaminathan et al., 2016; Spencer et al., 2018; Li et al., 2019). However, contractile patterning of the caecum (the equivalent of the human appendix) is not well characterised. Caecal motility has been described in studies of chicken (Janssen et al., 2009; van Staaveren et al., 2020), guinea pig (Schulze‐Delrieu et al., 1996), and rabbit (Hulls et al., 2012 and 2016), but is yet to be modelled in mice or studied in humans.
GI dysmotility is a common comorbidity in many disorders including autism spectrum disorder (ASD; autism) (Gorrindo et al., 2012; McElhanon et al., 2014; Vuong and Hsiao, 2017; Lee et al., 2020) and Parkinson’s disease (Kupsky et al., 1987; Singaram et al., 1995; Cersosimo et al., 2013; Giancola et al., 2017). Given that changes in gut motility patterns can contribute to gastrointestinal symptoms that impact quality of life, such as constipation, diarrhoea, visceral pain, and/or bacterial overgrowth, the accurate measurement of motility patterns could assist in identifying potential therapeutic targets to treat these issues. It is therefore necessary to design and utilise unbiased methods to study gut motility patterns both to gain fundamental knowledge about the neural regulation of motility and to determine changes in these patterns in pre-clinical animal models of disease. Ex vivo motility assays enable the investigation of gut motility patterns in animal models (Roberts et al., 2007; Swaminathan et al., 2016). These techniques were first developed to investigate small intestinal peristalsis in guinea pigs (Hennig et al., 1999; Gwynne and Bornstein, 2007) and then expanded for use in the mouse colon (Swaminathan et al., 2016). This method has been primarily applied to segments of mouse and guinea pig colon (Roberts et al., 2007; Hosie et al., 2019; Leembruggen et al., 2020) and, in some studies, to segments of small bowel (Hennig et al., 1999; Gwynne et al., 2004; Neal et al., 2009). As mentioned previously, studies of caecal motility are restricted to rabbit and chicken (Schulze‐Delrieu et al., 1996; Janssen et al., 2009; Hulls et al., 2012 and 2016). Even though external innervation from the central nervous system (CNS) plays a role in modulating gut motility (Browning and Travagli, 2014), the ENS is capable of independently regulating GI function. Hence, a major advantage of the ex vivo video imaging technique reported here is the ability to measure gut motility in the absence of CNS inputs.
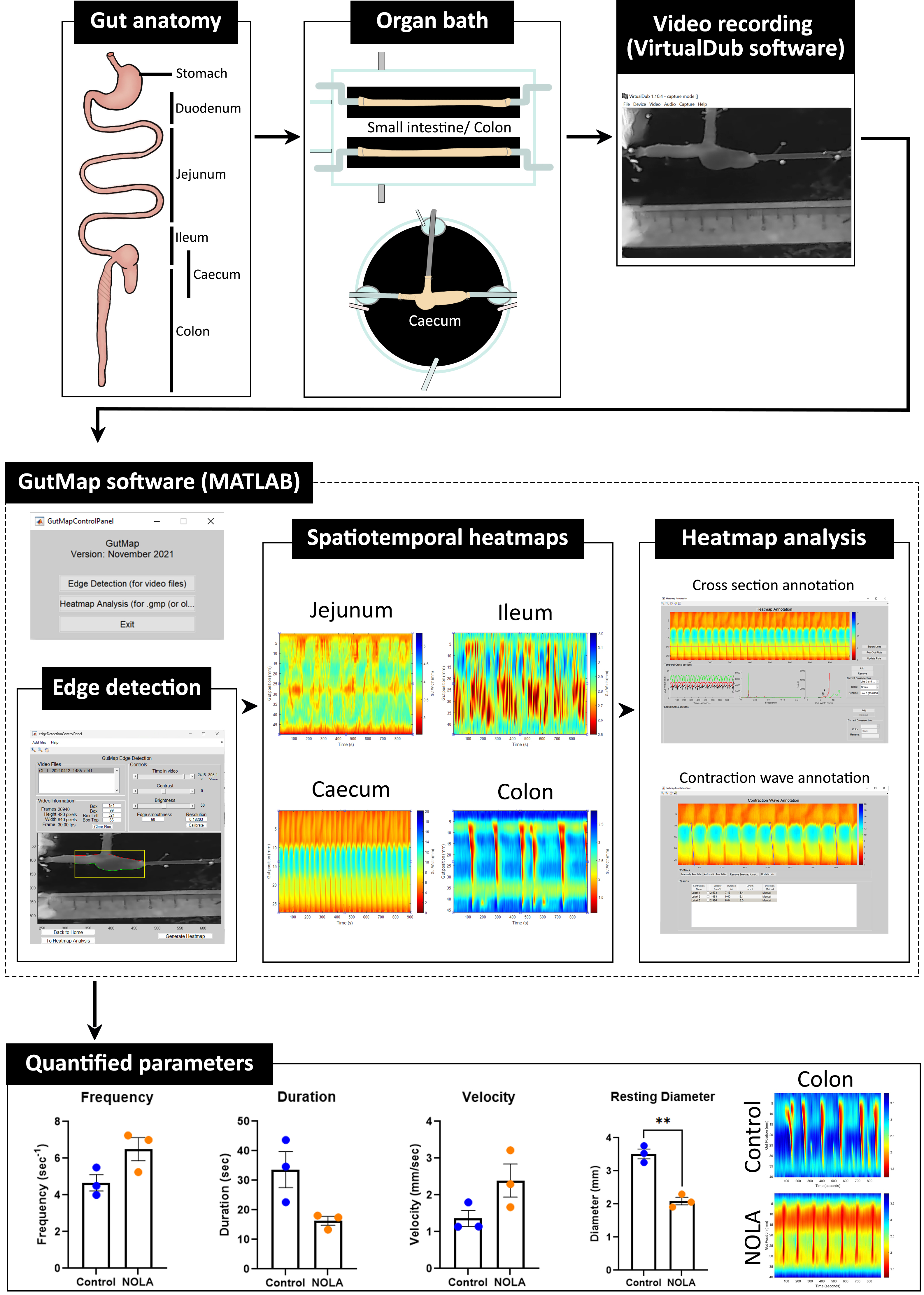
Video imaging combined with spatiotemporal mapping enables the quantitative assessment of gastrointestinal motility in animal models. Using these techniques, multiple contractility parameters such as propagation speed, magnitude, length, duration, diameter, and frequency of gastrointestinal contractions can be measured. However, the software platform we previously utilised for this purpose (Swaminathan et al., 2016) had limited functionality. A lack of compatibility with current software platforms and closed source code that prevented flexibility were major limitations. We found that although suitable for assessing intestinal motility patterns, the edge detection function within the previous version of the MATLAB-based software (Analyse2) could not accurately detect edges of tissue segments from irregular shaped gut regions, such as the caecum, due to the curved/nonlinear anatomy of this organ. In addition, the quality of the heatmaps generated using the previous edge detection function (Swaminathan et al., 2016) was lower, as 16-bit unsigned integer arrays led to a quantisation and pixelation of the resultant spatiotemporal heatmaps. Importantly, this edge detection code was not compatible across different operating systems (i.e., Windows and Macintosh), which reduced user accessibility. Here, we highlight the utility of the new MATLAB-based software interface, entitled GutMap, that enables i) accurate edge detection in multiple gut regions, including in combination with a novel mouse caecal motility protocol (which can additionally be applied to stomach motility), ii) output of high-resolution spatiotemporal heatmaps, and iii) usage across different operating systems.
GutMap is a user-friendly enhanced software interface (available on request) that enables a sensitive and robust approach for visualising and analysing regional gut motility patterns in rodents (with potential applications in other preclinical models following some modification). Data from video recordings of gut contractility patterns acquired from different regions of the GI tract (i.e., the small intestine, caecum, and colon) are converted to high-resolution spatiotemporal heatmaps. The GutMap resolution, measured in μm/pixel or mm/pixel as appropriate to the length scale of the experimental preparation, is entirely dependent on the resolution of the input video file. The spatiotemporal heatmaps generated are high quality as the edge data is stored in double precision arrays, providing a near-continuum of edge locations and thus greater precision of gut width measurements.
Spatiotemporal heatmaps display gut diameter and gut position plotted as a function of time and assign warm colours denoting gut contractions and cool colours indicating gut relaxation. GutMap can be used to measure multiple contractile parameters to characterise subtle changes in motility patterns and ENS activity. Novel features of GutMap include a video calibration function, real-time video tagging information that shows the video properties and edge detection dimensions, as well as a file queueing function for edge detection processing. These features ensure increased accuracy and consistency of measures (i.e., based on the initial tissue size calibration) for each video recording file, as well as streamlined heatmap generation. Heatmap analysis using GutMap enables full functionality of the previous Analyse2 module (Swaminathan et al., 2016), plus an additional novel analysis function that enables measurement of overall contractility of the gut tissue segment.
Here, we provide a comprehensive guide for investigating ex vivo gastrointestinal motility in rodent models and highlight the novelty of our caecal motility measurement protocol in female mouse GI preparations, using the novel video analysis software, GutMap. A detailed explanation is provided for the experimental setup and execution, which are adapted from studies performed in mouse colon (Swaminathan et al., 2016) and rabbit caecum (Hulls et al., 2012 and 2016). Here, we also outline detailed steps for generating and analysing spatiotemporal heatmaps using GutMap. We demonstrate that these processes enable the comparison of motility patterns in different gut regions (i.e., the small intestine, caecum, and colon) both in physiological conditions and in response to drug administration [i.e., the nitric oxide synthase (NOS) inhibitor drug, Nω-Nitro-L-arginine (NOLA)].
The method can be further extended for use in various conditions including examining gut motility in response to different treatments and in other preclinical models of disease.
Materials and reagents
Two-chamber organ bath [manufactured by The University of Melbourne, Department of Physics (Swaminathan et al., 2016)] or equivalent, such as glass Petri dish, 14 cm diameter (e.g., Sterilin 140 mm Petri dish, Thermo Scientific, catalog number: 501V)
Organ bath tubing
Krebs solution inflow tubes
i. Polyethylene tubing 3.00 mm × 2.00 mm (Microtube Extrusions, North Rocks NSW, catalog number: PE300200)
ii. 1 cm of Masterflex Platinum-cured silicone tubing (John Morris Group, catalog number: 96410-14; L/S 14, 25 ft)
iii. Silicone rubber tubing 0.078" ID × 0.125" OD × 50 ft Sil-Med Corporation
iv. Tube connector (200 μL pipette tip) (Axygen, catalog number: AX-T-200-Y)
Vacuum and carbogen tubes
Laboratory tubing 1.02 mm ID × 2.16 mm OD (Silastic, catalog number: 508-005)
Front and back pressure cannulation inflow tubes
i. Single lumen polyethylene tube OD 2.00 mm × ID 1.00 mm used as cannula (catalog number: 112074)
ii. 0.2 cm of laboratory tubing 1.02 mm ID × 2.16 mm OD to create grip at the end of cannula (Silastic, catalog number: 508-005)
iii. Masterflex L/S® precision pump tubing, platinum-cured silicone (John Morris Group, catalog number: L/S 16 96410-16) to connect to 3-way stopcock and silicone tube connector
iv. Masterflex L/S® precision pump tubing, platinum-cured silicone (John Morris Group, catalog number: L/S 14 96410-14) to connect cannula and tube connector
v. Tube connector (200 μL pipette tip) (Axygen, catalog number: AX-T-200-Y)
Circulating water bath tubes
i. Platinum-cured silicone Masterflex L/S® precision pump tubing (John Morris Group, catalog number: L/S 15 96410-15)
Luer Lock tip syringe, 60 mL (Livingstone, catalog number: DSL050MLLCL)
Luer Slip tip tuberculin syringe, 1 mL (Livingstone, catalog number: DSL001MLSC)
Luer Slip tip syringe, 10 mL (Livingstone, catalog number: DS100MTL)
Rubber stopper (Mad About Science, 10 pack, SKU: MAS-03121-1)
Capillary glass tubing, 8 mm diameter, 1.5 mm wall thickness
Multi-purpose sealant [732 Dow Corning (clear) or equivalent]
Suture thread (white cotton thread, Woolworths Supermarket, Woolworths Group, Australia)
Nω-Nitro-L-arginine (NOLA) (Sigma-Aldrich, catalog number: N5501-5G)
Sylgard
184 Silicone elastomer base (DOWSIL)
184 Silicone elastomer curing agent (DOWSIL)
3-way stopcocks (B Braun Discofix, catalog number: 16494C)
Glass media bottle, clear, 500 mL with hose connection at base
Activated charcoal (White Glo)
Filter flask 2 L (Kimax Kimble, catalog number: 27060)
Sodium chloride (NaCl) (Chem-Supply, catalog number: SA046-5KG)
Potassium chloride (KCl) (Ajax Finechem, catalog number: AJA383-500G)
Sodium dihydrogen orthophosphate dihydrate (NaH2PO4·2H2O) (Chem-Supply, catalog number: SA328-500G)
Magnesium sulphate heptahydrate (MgSO4·H2O) (Chem-Supply, catalog number: MA048-500G)
Calcium chloride dihydrate (CaCl2·2H2O) (Ajax Finechem, catalog number: 127-500G)
D-glucose anhydrous (Chem-Supply, catalog number: GA018-500G)
Sodium hydrogen carbonate (NaHCO3) (Chem-Supply, catalog number: SA001-5KG)
Hydrochloric acid (HCl) (Merck Millipore, SKU: 100313)
Distilled H2O (dH2O)
Krebs 10× stock solution in 2 L (4 °C) (see Recipes)
1× Krebs solution in 2 L (4 °C) (see Recipes)
NOLA solution 100 mM (4 °C) 5 mL (see Recipes)
100 μM NOLA-Krebs solution in 500 mL (see Recipes)
Recipes
Krebs 10× stock solution in 2 L (4 °C)
Reagent Final concentration Quantity Sodium chloride (NaCl) 1.18 M 138 g Potassium chloride (KCl) 47.6 mM 7.1 g Sodium dihydrogen orthophosphate dihydrate (NaH2PO4·2H2O) 10 mM 3.1 g Magnesium sulphate heptahydrate (MgSO4·H2O) 12 mM 5.9 g Calcium chloride dihydrate (CaCl2·2H2O) 32.99 mM 9.7 g dH2O n/a 2 L 1× Krebs solution in 2 L (4 °C)
Reagent Final concentration Quantity Krebs 10× stock solution 10% 200 mL dH2O n/a 2 L D-glucose anhydrous 11.1 mM 4.0 g Sodium hydrogen carbonate (NaHCO3) 25 mM 4.2 g NOLA solution 100 mM (4 °C) 5 mL
Reagent Final concentration Quantity Nω-Nitro-L-Arginine (NOLA) 100 mM 109.6 mg Hydrochloric acid (HCl) 1 M 400 μL dH2O n/a 4.6 mL 100 μM NOLA-Krebs solution in 500 mL
Reagent Final concentration Quantity NOLA solution 100 μM 500 μL 1×Krebs l solution n/a 500 mL
Equipment
Webcam (Logitech Carl Zeiss Tessar HD 1080p or equivalent)
Dry block heater (Ratek, model PB)
Circulating water bath 8.5 L (ELMI TW-2.03)
Carbogen, BOC Limited
Vacuum (e.g., laboratory vacuum system)
Retort-stand with clamp (e.g., Australian Scientific, catalog number: SKU: 91055006, 91055028, 120015)
Dumont tweezer, style 1 (Fine Science Tools, catalog number: T01-111)
Dumont forceps, style 5 (Fine Science Tools, catalog number: 11252-30)
Curved forceps (Fisher Scientific, catalog number: 17-467-201)
Fine dissecting scissors (Fine Science Tools, catalog number: 14060-09)
Vannas spring scissors (Fine Science Tools, catalog number: 15000-08)
Amber scintillation 250 μL glass vial (Agilent, catalog number: 5183-2085) for light-sensitive NOLA solution 100 mM
Insect pins (Australian Entomological Supplies, catalog number E-157)
Thermometer (Westlab, catalog number: 072002-0131)
Software
VirtualDub version 1.10.4 64-bit
GutMap (must be downloaded into the MATLAB program files folder)
MATLAB 2021b (or any version of MATLAB that is compatible with Mac or Windows devices):
Curve fitting toolbox
Image processing toolbox
Logitech HD Pro Webcam C920 Driver
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Abo-Shaban, T., Lee, C. Y. Q., Hosie, S., Balasuriya, G. K., Mohsenipour, M., Johnston, L. A. and Hill-Yardin, E. L. (2023). GutMap: A New Interface for Analysing Regional Motility Patterns in ex vivo Mouse Gastrointestinal Preparations. Bio-protocol 13(19): e4831. DOI: 10.21769/BioProtoc.4831.
分类
生物信息学与计算生物学
生物工程 > 生物医学工程
神经科学 > 周围神经系统
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









