- EN - English
- CN - 中文
Intraperitoneal Injection of Neonatal Mice
新生小鼠腹腔注射
(*contributed equally to this work) 发布: 2023年09月20日第13卷第18期 DOI: 10.21769/BioProtoc.4826 浏览次数: 3300
评审: Vivien J. Coulson-ThomasSudhir VermaAnonymous reviewer(s)
Abstract
Administration of substances into neonatal mice is required for early treatment with pre-clinical therapeutics, delivery of recombination-inducing substances, and dosing with viruses or toxins, amongst other things. Several injection routes into mouse pups are possible, including intravenous and intracerebroventricular, each with their own advantages and limitations. Here, we describe a simple and rapid protocol for the intraperitoneal injection of neonatal mice for systemic dosing. By detaching a 30-gauge needle from its plastic hub and inserting it into polyethylene tubing attached to a Hamilton syringe, small volumes (1–10 μL) can be accurately injected into the peritoneal cavity of pups aged 1–5 days old. The procedure can be completed within a few minutes, is generally safe and well tolerated by both pups and parents, and can be used in combination with alternative administration routes.
Key features
• This protocol provides a simple description to rapidly and efficiently inject mouse pups aged 1–5 days for systemic dosing.
• Allows treatment of neonatal mice with substances such as viruses and compounds for research across disciplines.
Graphical overview
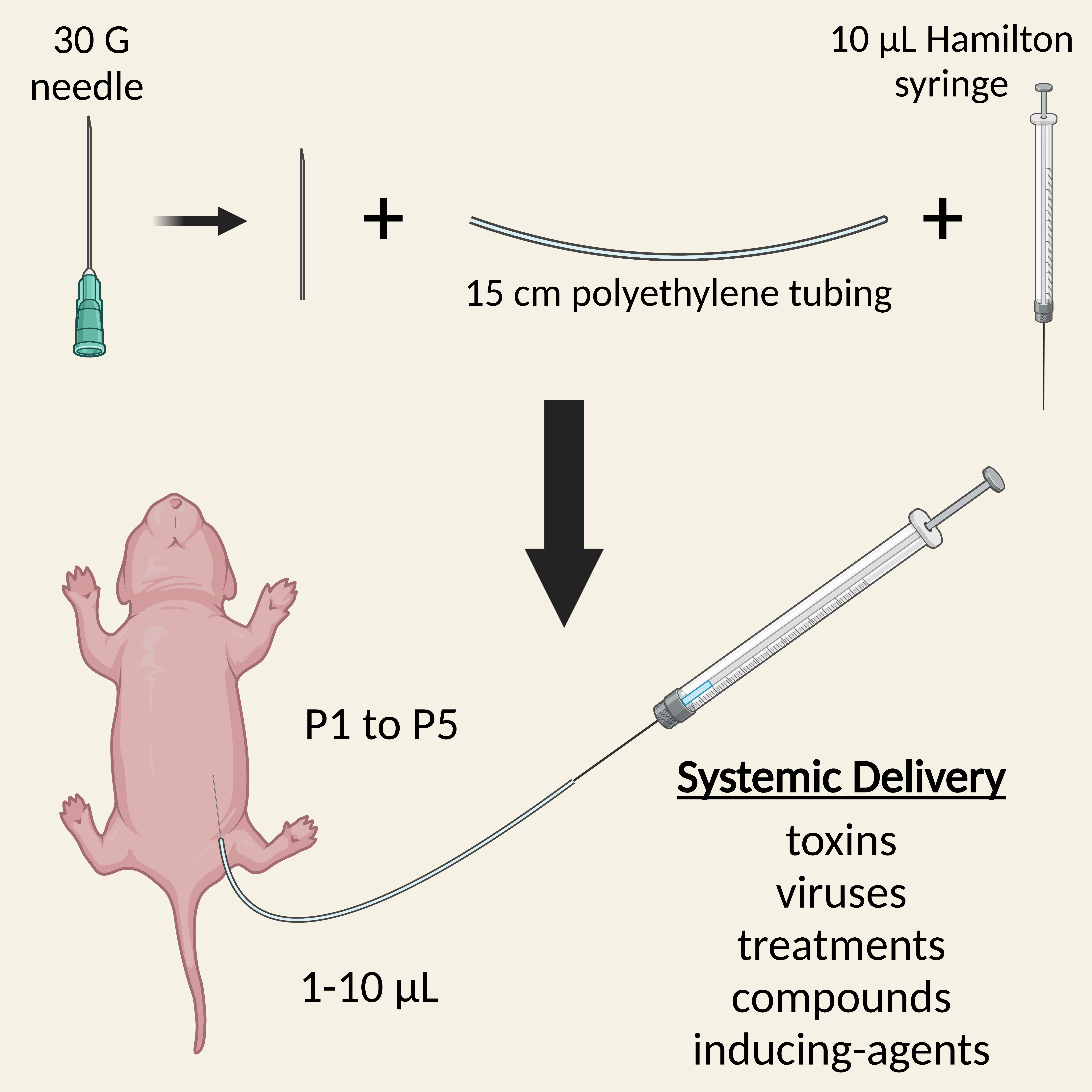
Background
The early post-natal injection of substances into rodents is required for several purposes, including the testing of pre-clinical therapeutics in disease models (Hua et al., 2011), delivering recombination-inducing substances such as tamoxifen (Pitulescu et al., 2010), and performing mechanistic studies with viruses or toxins (MacDonald et al., 2021). Several delivery routes into neonates are available, each with advantages and pitfalls. For instance, injection into the cerebral lateral ventricles (i.e., intracerebroventricular) can bypass the blood–brain barrier and deliver substances throughout the central nervous system via the cerebrospinal fluid, but this injection paradigm will not result in systemic delivery, is not suitable for repetitive treatments, and is technically challenging (Passini and Wolfe 2001). Alternatively, intravenous injections permit greater volumes (~50–100 μL) and result in a more systemic effect; however, this route is tricky to master (Sands and Barker 1999; Kienstra et al., 2007) and becomes more difficult in pups older than two days due to vein visualisation issues (Gombash Lampe et al., 2014). Subcutaneous injections are straightforward and enable larger dosing volumes if needed, but rates of substance absorption are slower compared to other delivery routes (Turner et al., 2011). Intraperitoneal neonatal injections also allow body-wide substance administration via transfer to the circulatory system (thus rapid absorption) and require less expertise and technical ability but are limited by injection volume and are not frequently used in a clinical setting (Al Shoyaib et al., 2019).
Nevertheless, different injection routes into neonatal rodents can result in distinct tissue targeting (Ohshima et al., 2015) and varied temporal pharmacodynamics post-injection (Statler et al., 2007; Foust et al., 2008); Therefore, it is important to master a variety of administration strategies. Indeed, depending on the dosing and targeting requirements, combining injection paradigms in pups is possible and can provide a superior therapeutic effect compared to single-route injections (Nizzardo et al., 2014).
Several published methods for the delivery of substances into neonates via intravenous (temporal/facial vein, jugular vein, or retro-orbitally) and intracerebroventricular routes are available (Sands and Barker 1999; Kienstra et al., 2007; Glascock et al., 2011; Yardeni et al., 2011; Gombash Lampe et al., 2014). However, a similar, detailed protocol for the intraperitoneal injection of mouse neonates is lacking, although approaches have been presented in brief (Ostermann et al., 2013; Xu et al., 2018; MacDonald et al., 2021).
Here, we describe a stepwise method for accurate injections into the peritoneal cavity of mouse pups, which has been used to successfully deliver adeno-associated viruses to drive transgene expression in 1–3-day-old mice (MacDonald et al., 2021; Sleigh et al., 2023). Intraperitoneal injections are highly reproducible and easy to master and can be used for repeated treatments. Success rates will depend on experimenter experience and the substances being injected, but our recent experiments suggest that a near 100% success rate is achievable.
Materials and reagents
Note: Similar materials, reagents, and equipment can be purchased from alternative sources. We note that alternative sourcing of 0.28 mm internal diameter tubing may lead to issues in creating a watertight seal with the 30 G needle (e.g., variance in actual vs. reported internal diameter).
Cannula fabrication
Disposable surgical scalpel (Swan Morton, VWR International, catalog number: 0505)
PE-10/10 Polyethylene tubing, 0.28 mm internal diameter, 0.61 mm external diameter (Warner Instruments, Multi-Channel Systems, catalog number: 64-0750)
30 G × 1/2" needle (BD, catalog number: 305106)
6-well plate, flat bottom (VWR International, catalog number: 734-2777)
Sterile water
10 mL syringe (Fisherbrand, Fisher Scientific, catalog number: 15879152)
10 μL syringe, 701 N, 26 G, 51 mm length, 0.47 mm external diameter (Hamilton, VWR International, catalog number: 549-1135)
Intraperitoneal injection
Disposable surgical drape, e.g., 30 cm × 45 cm (Millpledge, catalog number: SDT0100)
Large weigh boat (Fisherbrand, Fisher Scientific, catalog number: 15758187)
Biohazard sharps bin
Cannula (see above)
Substance(s) for injection (e.g., virus, toxin, compound) in 1.5 mL microfuge tube(s) on ice or disposable heating pad, as needed
Sterile cotton swab (Texwipe, Fisher Scientific, catalog number: 15823886)
Marker pen
P20 pipette tips
Parafilm (VWR International, catalog number: 291-0057)
Paper towel or surgical gauze
Optional:
50 mL centrifuge tube (Falcon, Fisher Scientific, catalog number: 10788561) with 20–40 mL of bleach (for viruses/toxins)
Insulin syringe (BD, Fisher Scientific, catalog number: 13161931) (for toe tattooing)
Tattoo ink (Ketchum, VWR International, catalog number: NASCC01885N) (for toe tattooing)
Equipment
Cannula fabrication
30 cm ruler or tape measure
Straight tip haemostat, Stainless steel, 5" (Newport Spectra-Physics, catalog number: LAB-17) (see Note 1)
Intraperitoneal injection
Animal heating pad (Tonkey Electrical Technology, catalog number: TK-HPP4030) or disposable heating pad (e.g., HotHands Hand Warmers) (see Note 2)
10 μL syringe, 701 N, 26 G, 51 mm length, 0.47 mm external diameter (Hamilton, VWR International, catalog number: 549-1135) (see Note 3)
P20 pipette
Optional:
Class II biosafety cabinet (for viruses/toxins)
Rack for 50 mL centrifuge tubes (for viruses/toxins)
Magnifying light (to aid injection accuracy)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Pocratsky, A. and Sleigh, J. N. (2023). Intraperitoneal Injection of Neonatal Mice. Bio-protocol 13(18): e4826. DOI: 10.21769/BioProtoc.4826.
分类
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
提问指南
+ 问题描述
写下详细的问题描述,包括所有有助于他人回答您问题的信息(例如实验过程、条件和相关图像等)。
Share
Bluesky
X
Copy link










