- EN - English
- CN - 中文
Double Staining with Fluorescent Tracers to Determine Myeloid Cell Migration of Leishmania-infected Cells from Mouse Skin to Lymphatic Tissues by Flow Cytometry
用荧光示踪剂双重染色通过流式细胞术测定利什曼原虫感染细胞从小鼠皮肤到淋巴组织的骨髓细胞迁移
发布: 2023年09月20日第13卷第18期 DOI: 10.21769/BioProtoc.4817 浏览次数: 2308
评审: Alexandros AlexandratosMaria AgallouAnonymous reviewer(s)
Abstract
Immune cell trafficking in steady-state conditions and inflammatory cell recruitment into injured tissues is crucial for the surveillance of the immune system and the maintenance of body homeostasis. Tracking the cell journey from the infection site in the skin to lymphoid tissues has been challenging, and is typically determined using fluorescent cell tracers, antibodies, or photoconvertible models. Here, we describe the detailed method to track Leishmania-infected myeloid cells migrating from the skin to lymphatic tissues by multiparametric flow cytometry. These methods involve labeling of infective Leishmania donovani parasites with fluorescent cell tracers and phenotyping of myeloid cells with fluorescent antibodies, to determine the infection status of migratory myeloid cells. We also describe the detailed protocol to trace donor monocytes transferred intradermally into recipient mice in Leishmania donovani infection. These protocols can be adapted to study skin-lymphoid tissue migration of dendritic cells, inflammatory monocytes, neutrophils, and other phagocytic myeloid cells in response to vaccine antigens and infection.
Key features
• Cell-tracking of cell-trace-labeled parasites and monocytes from the skin to lymphatic tissues after transference into donor mice.
• Identification of migratory cells labeled with fluorescent cell tracers and antibodies by flow cytometry.
• Isolation, labeling, and transference of bone marrow monocytes from donor mice into the skin of recipient mice.
• Description of a double-staining technique with fluorescent cell tracers to determine cell and parasite dissemination from the skin to lymphoid tissues.
Graphical overview
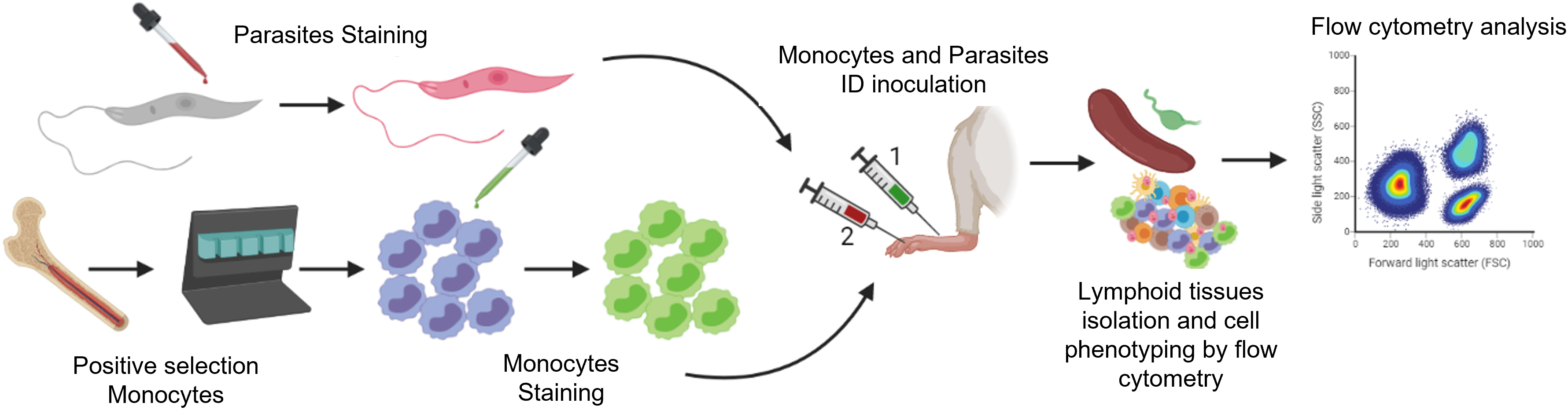
Overview of the methods to trace the migration of Leishmania and monocytes from the skin to lymphatic tissues by flow cytometry. Infective metacyclic promastigotes (from axenic culture) and monocytes (isolated from the bone marrow of donor mice) are labeled with fluorescent cell tracers. After intradermal injection into the test mouse (1, 2), migratory cells and infected cells are isolated from the skin and lymphoid tissues of the test mouse. These cells are then labeled with fluorescent antibodies against myeloid cells and recognized according to the differential excitation/emission wavelengths of the fluorochromes by flow cytometry.
Background
The migration of immune cells is a fundamental function of the immune system in health and disease. Immune cell movement through the lymphatic network plays a crucial role in immune surveillance, the initiation of the immune response, and the development of tolerance. Innate immune cells, such as neutrophils, macrophages, and dendritic cells capture antigens and capture and kill pathogens, activating the adaptative immune response at the draining lymph node (Hampton and Chtanova, 2019). However, intracellular parasites have also developed strategies to circumvent the immune response, survive, and disseminate through the lymphatics to other tissues (Hampton and Chtanova, 2019).
Cell migration can be studied by diverse approaches with different drawbacks. Time-lapse microscopy tracks short fragments of the individual cell migration and generates large amounts of digital data, making the analysis complicated, even with access to software tools for automated cell tracking (Al-Zaben et al., 2019). Intravital microscopy (IVM) is useful for the visualization of cell trafficking, proliferation, and cell death at single-cell resolution level (Maiorino et al., 2022). However, IVM is an invasive method that can generate local inflammation and tissue damage (Maiorino et al., 2022). Most IVM techniques are based on fluorescence microscopy, but in the last two decades, different biological processes have been explored using IVM together with two/three-photon microscopy to avoid dye use. However, the long duration of image acquisition can cause photodamage and an inadequate spatial co-registration between sequentially detected signals (You et al., 2018). Currently, photoconvertible transgenic mice are being used for cell migration studies; however, the establishment of photoactivation conditions is tedious, requiring the coupling of the photoactivation technique and instrumentation for signal detection. The most popular photoactivation/conversion model, Kaede mice, helps to understand cell migration at single-cell level or by invasive intracranial imaging. However, this technique is not suitable for models involving photoactivation of cells in the skin or for studies requiring deep tissue photoactivation (Tomura et al., 2021). Similarly, in our hands, the photoactivable model R26-PAGFP-LysMcre [B6.Cg-Gt(ROSA)26Sortm1(CAG-PA-GFP)Rmpl/J mice mated with B6.129P2-Lyz2tm1(cre)Ifo/J] was not suitable for identifying migratory cells photoactivated in the skin (Figure S1, Video S1 and Table S1). Therefore, fluorescent cell tracers are the most inexpensive, straightforward technique to study cell migration from skin to lymphatic organs.
Fluorescent cell trackers are powerful tools that enable the direct visualization of biological processes, such as cell proliferation, cell migration, and cell–cell interactions (Halabi et al., 2020). Thus, it is possible to stain cells or pathogens with fluorescent tracers to follow their migration through the lymphoid tissues of the host. The use of cell trackers coupled with flow cytometry makes it possible to identify the infected cells and determine the cell populations carrying parasites from the skin (Ibrahim et al., 2013). The higher specificity, sensitivity, and robustness of this method are reached by the combination with flow cytometry. The protocol described here was applied to the study of infection status and migration of innate immune cells (monocytes, dendritic cells, and neutrophils) to lymph node and spleen of after-skin-infection with Leishmania spp. (Osorio et al., 2023).
Materials and reagents
Biological materials
Balb/c mice (4–7 weeks old) (Envigo, Inotiv)
Wildtype or mCherry Leishmania donovani metacyclic promastigotes (IS strain; MHOM/SD/00/S-2D) (Osorio et al., 2023)
Reagents
RPMI 1640 medium (Thermo Fisher Science, GibcoTM, catalog number: 11875093)
M199 medium (Thermo Fisher Science, GibcoTM, catalog number: 11150067)
Penicillin-streptomycin solution (Thermo Scientific, GibcoTM, catalog number: 15140163)
Fetal bovine serum (FBS), inactivated by heating at 56 °C for 20 min (Thermo Scientific, GibcoTM, catalog number: 16000044)
0.4% Trypan Blue solution (Sigma-Aldrich, catalog number: T8154)
Adenine (Sigma-Aldrich catalog number: A8626)
Hemin (Millipore, Sigma, catalog number: H9039)
HEPES buffer solution 1 M (Millipore, Sigma, catalog number: 83264)
Sodium chloride (NaCl) (Millipore, Sigma, catalog number: S9888)
Potassium chloride (KCl) (Millipore, Sigma, catalog number: P3911)
Magnesium chloride hexahydrate (MgCl2·6H2O) suitable for cell culture (Millipore, Sigma, catalog number: M2393)
Calcium chloride (CaCl2) suitable for cell culture (Millipore, Sigma, catalog number: C7902)
Collagenase D (Millipore, Sigma, Roche, catalog number: 11088858001)
DNase I (Millipore, Sigma, Roche, catalog number: 10104159001)
LiberaseTM TL (Millipore, Sigma, Roche, catalog number: 5401020001)
Accutase cell detachment solution (STEMCELL Technologies, catalog number: 07920)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7030)
10× phosphate-buffered saline (PBS) (Corning®, catalog number: 46-013-CM)
Dimethyl sulfoxide (DMSO) (hybridoma grade) (Sigma-Aldrich, catalog number: D2650)
MojoSortTM mouse Ly-6G Selection kit (BioLegend, catalog number: 480123)
MojoSortTM buffer (5×) (BioLegend, catalog number: 480017)
MojoSortTM magnet (BioLegend, catalog number: 480020)
Anti-CD11b magnetic particles DM Clone M1/70 (RUO) (BD IMagTM, catalog number: 558013)
Lectin from Arachis hypogaea (peanut) PNA agglutinin (Sigma-Aldrich, catalog number: L-08881)
Dibutyl phthalate (Sigma-Aldrich, catalog number: 524980)
1-step Fix/Lyse solution (10×) (eBioscienceTM, catalog number: 00533354)
BD IMagTM buffer (10×) (BD, catalog number: 552362)
RBC lysis buffer (10×) (BioLegend, catalog number: 420301)
TruStain FcXTM (anti-mouse CD16/32) antibody (BioLegend, catalog number: 101320)
Fixable Viability Dye eFluor 455UV (eBioscienceTM, catalog number: 65-0868)
Antibodies for flow cytometry (see Table 1)
Cell tracer dyes (see Table 2)
Table 1. Antibodies and probes for ex vivo staining of myeloid cells by flow cytometry
Antibody name (Concentration of stock) Ex/Em (nm) Working concentration
μg/tube (μL)
Clone Brand and catalog number Anti-mouse CD3- FITC (0.5 mg/mL) 495/519 1.0 (0.25) 17A2 BioLegend, 100204 Anti-mouse CD19-FITC (0.5 mg/mL) 495/519 0.125 (0.25) 1D3/CD19 BioLegend, 152404 Anti-mouse NK-1.1-FITC (0.5 mg/mL) 495/519 0.25 (0.25) PK136 BioLegend, 108706 Anti-mouse CD45-PE-Cy7 (0.2 mg/mL) 488, 532, 561/767 0.125 (0.5) 30-F11 BioLegend, 103114 Anti-mouse/human CD11b-eFluor 506
(0.2 mg/mL)
419/508 0.125 (0.5) M1/70 eBioscience, 69-0112-82 Anti-mouse Ly6C-BV 785 (0.2 mg/mL) 405/785 0.25 (0.5) HK1.4 BioLegend, 128041 Anti-mouse Ly6G–APC-Cy7 (0.2 mg/mL) 650, 755/767 0.25 (0.5) 1A8 BioLegend, 127624 Anti-mouse CD11c-BV 605 (0.2 mg/mL) 405/605 0.25 (1) N418 BioLegend, 117334 Viability (Fixable Viability Dye-eFluor 455) (100 test) 355/450 0.1 (0.01) NA eBioscience, 65-0868 Note: Antibody titration is recommended for optimal performance. Avoid direct exposure of labeled samples to light. Acquire samples in a flow cytometer as soon as possible to one week at 4 °C (better within 24 h). Use FMOs, unstained control (no-cell trace control tissues) for gating. For myeloid cell phenotyping, use fluorochromes compatible with your flow cytometer. Use FluoroFinder or other software for panel design (to avoid spectral overlap of fluorescence based on the configuration of your flow cytometer).
Table 2. Cell tracer dyes used in the study; the listed dyes can be used to track cells or parasites*
Cell tracer name
(Concentration of stock)
Storage Excitation/Emission (nm) Final concentration Brand and catalog number CellTrackerTM Orange CMTMR (10 mM) -20 °C 541/565 nm 2.5 μM Invitrogen C2927 CellTrackerTM Green CMFDA (10 mM) -20 °C 492/517 nm 5 μM Invitrogen C2925 CellTraceTM Far-Red (1 mM) -20 °C 630/661 nm 1 μM Invitrogen C34572 PKH26 Red Fluorescent Cell Linker 4 °C 551/567 nm 4 μM Sigma-Aldrich MINI26-1KT PKH 67 Green Fluorescent Cell Linker 4 °C 490/502 nm 4 μM Sigma-Aldrich MINI67-1KT *mCherry (5871/610 nm)
Solutions
1× phosphate-buffered saline (1× PBS) (see Recipes)
1× PBS with 0.1% BSA: FACS buffer (see Recipes)
1× PBS + 2% FBS (see Recipes)
1× 1-Step Fix/Lyse solution (see Recipes)
1× RBC lysis buffer (see Recipes)
1× BD IMagTM buffer (see Recipes)
Complete M199 medium (see Recipes)
Complete RPMI 1640 medium (see Recipes)
Collagenase D + Liberase TL Mix (see Recipes)
Collagenase D solution + DNase I (see Recipes)
Recipes
1× phosphate-buffered saline (1× PBS) from 10× PBS (4 °C storage)
Reagent Final concentration Quantity 10× PBS 1× 100 mL Distilled H2O n/a 900 mL Total n/a 1,000 mL 1× PBS with 0.1% BSA. FACS buffer (4 °C storage)
Reagent Final concentration Quantity 1× PBS 1× 1,000 mL BSA 0.1% 1 g Total n/a 1,000 mL 1× PBS + 2% FBS (4 °C storage)
Reagent Final concentration Quantity 1× PBS 1× 980 mL 100% FBS 2% 20 mL Total n/a 1,000 mL 1× 1-Step Fix/Lyse solution (4 °C storage)
Reagent Final concentration Quantity 10× 1-step Fix/Lyse 1× 100 mL Distilled H2O n/a 900 mL Total n/a 1,000 mL 1× RBC lysis buffer (4 °C storage)
Reagent Final concentration Quantity 10× RBC lysis buffer 1× 10 mL Distilled H2O n/a 90 mL Total n/a 100 mL 1× BD IMagTM buffer (4 °C storage)
Reagent Final concentration Quantity 10× BD IMagTM buffer 1× 10 mL Distilled H2O n/a 90 mL Total n/a 100 mL Complete M199 medium (4 °C storage)
Reagent Final concentration Quantity M199 medium n/a 500 mL FBS 10% 50 mL Adenine 1% 6 mL Hemin 0.1% 1.2 mL Penicillin-streptomycin solution 1× 6 mL Total n/a 563.2 mL Complete RPMI 1640 medium (4 °C storage)
Reagent Final concentration Quantity RPMI medium n/a 445 mL FBS 10% 50 mL Penicillin-streptomycin solution 1× 5 mL Total n/a 500 mL Collagenase D + Liberase TL Mix (storage at -20 °C)
Reagent Final concentration Quantity 20 mg/mL Collagenase D 2 mg/mL 2 mL 1 mg/mL Liberase TL 160 μg/mL 640 μL RPMI complete medium n/a 17.36 mL Total n/a 20 mL Collagenase D Solution + DNase I (storage at -20 °C)
Reagent Final concentration Quantity 20 mg/mL Collagenase D 2 mg/mL 5 mL 2 mg/mL DNase I 20 μg/mL 500 μL 1 M HEPES pH 7.4 10 mM 500 μL 5 M NaCl 150 mM 1.5 mL 1 M KCl 5 mM 250 μL 0.5 M MgCl2 1 mM 100 μL 0.5 M CaCl2 1.8 mM 180 μL Distilled H2O n/a 42.5 mL Total n/a 50 mL
Laboratory supplies
Ultra-fine insulin syringe with needle (BD, catalog number: 324920)
Sterile 5 mL Polystyrene round bottom tube (Falcon, catalog number: 352054)
Sterile 70 μm cell strainer (any vendor)
Sterile 15 mL polypropylene centrifuge tubes (any vendor)
Sterile 50 mL polypropylene centrifuge tubes (any vendor)
Sterile nuclease-free filter tips (10, 200, and 1,000 μL) (any vendor)
Cell culture plates, different sizes, 6-, 12-, and 24-wells flat bottom, TC treated, sterile (any vendor)
Vented flask surface area 25 cm2 and 175 cm2 (any vendor)
Micro-dissecting scissors (Millipore, Sigma, catalog number: S3271)
Scissor-handle forceps (Millipore, Sigma, catalog number: Z168831)
Disposable plastic serological pipettes, sterile, individually wrapped, 10 mL, 25 mL capacity (any vendor)
Equipment
Flow cytometer with at least two lasers and seven parameter analysis capacity (BD LSRFortessa IITM Cell Analyzer, BD Biosciences; SE500, Stratedigm)
Eclipse TE300 Microscope (Nikon)
Software and datasets
Panel Builder, Fluorofinder (3, 2014, free)
FlowJo V10.6 software (V10.6, 2019, license required)
BD FACSDiva 9.0 (flow cytometer software)
GraphPad Prism 9.0 software (9, 2020, license required)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Uscanga-Palomeque, A. C., Osorio, E. Y. and Melby, P. C. (2023). Double Staining with Fluorescent Tracers to Determine Myeloid Cell Migration of Leishmania-infected Cells from Mouse Skin to Lymphatic Tissues by Flow Cytometry. Bio-protocol 13(18): e4817. DOI: 10.21769/BioProtoc.4817.
分类
免疫学 > 免疫细胞染色 > 流式细胞术
细胞生物学 > 细胞运动 > 细胞迁移
免疫学 > 免疫细胞分离 > 骨髓细胞
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link