- EN - English
- CN - 中文
A Large-format Polyacrylamide Gel with Controllable Matrix Mechanics for Mammalian Cell Culture and Conditioned Media Production
用于哺乳动物细胞培养和条件培养基生产的具有可控基质力学的大规格聚丙烯酰胺凝胶
发布: 2023年09月05日第13卷第17期 DOI: 10.21769/BioProtoc.4807 浏览次数: 2215
评审: Masashi AsaiXin XuAnonymous reviewer(s)
Abstract
Tissue culture plastic has been used for routine cell culture and in vitro experiments for over 50 years. However, cells are mechanically responsive and behave differently on hard surfaces than they do on softer substrates. Polyacrylamide gels have become a popular hydrogel of choice for controlling surface stiffness and ligand density for cell adhesion. Many synthesis methods use coverslips and small gel surface areas for cell culture, which are amenable to microscopy-based experiments. However, none of the currently published methods can be scaled up to increase the surface area to accommodate conditioned media production, high volume analyte collection, or cell line expansion. To overcome this size limitation, we developed a protocol for synthesizing polyacrylamide in glass dishes using commercially available materials. This enables routine cell culture on soft surfaces and facilitates experiments that require large amounts of analyte, especially studies involving extracellular vesicles and secreted factors.
Graphical overview
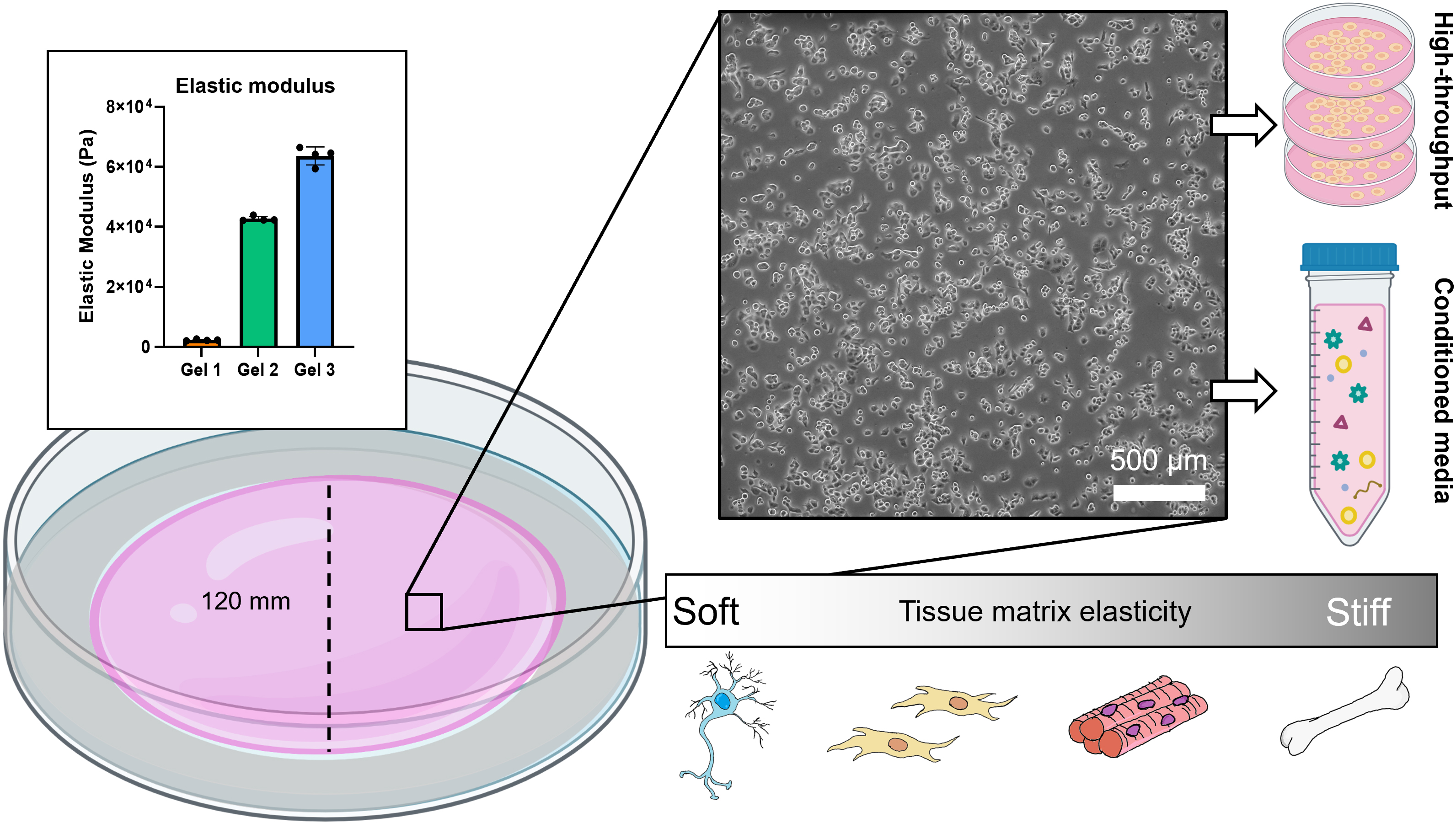
Background
Culturing mammalian cells on plastic is regarded as the gold standard—it is quick, easy, and inexpensive. However, the mechanical properties of plastic surfaces are not physiological, and many studies have demonstrated phenotypic, secretory, and transcriptional differences in cells cultured on compliant surfaces. Most tissues in the body are soft and have an elastic modulus (E) that falls between 1 and 100 kPa (Engler et al., 2006;Chandler et al., 2011;Gleghorn et al., 2013). Plastic is rigid and its elastic modulus measures on the order of Gigapascals (Chen and Simmons, 2011). Some cell lines are more sensitive to mechanical stimuli than others. Well established cell lines that have been cultured on plastic for many generations (e.g., NIH 3T3 fibroblasts) are less likely to be affected by routine culture as they have already been conditioned to grow on hard surfaces. Primary cells, which are isolated directly from fresh tissue, are susceptible to undesirable phenotypic and genotypic changes when they are placed in these altered mechanical environments. When cultured on plastic, cells may transition to an activated or inflammatory state, de-differentiate, or lose their primary function. Primary cells are thus passage-limited and have a relatively short expansion window before new cells must be used.
Matrix mechanics directly modulate cell signaling and function (Nelson and Gleghorn, 2012; Millar-Haskell et al., 2019; Morgan et al., 2019; Trompeter et al., 2021). This has been observed from in vitro experiments using a variety of hydrogels of different compositions and structure. Polyacrylamide gels are particularly useful because they are elastic, the stiffness can be tightly controlled by varying the concentrations of prepolymer, and a variety of extracellular matrix proteins can be conjugated to the gel for cell attachment. Polyacrylamide gel systems have yielded important observations regarding durotaxis (Lachowski et al., 2017), mechanotransduction (Stanton et al., 2019), and differentiation (Engler et al., 2006). Over the course of a decade, protocols have been published detailing the creation of polyacrylamide coverslips or platforms for cell-based experiments (Aratyn-Schaus et al., 2010;Tse and Engler, 2010; Mih et al., 2011; Syed et al., 2015; Kumai et al., 2021). These protocols present a standardized methodology, allowing experiments across research groups to be compared more easily. Generally, the process involves functionalizing a glass surface (e.g., coverslips or multi wells) to facilitate polyacrylamide attachment during polymerization. Sulfo-SANPAH is used to conjugate an extracellular matrix protein of choice to the polyacrylamide gel for cell attachment. Cells are then seeded on top of these gel surfaces for 24–72 h to study morphological and phenotypic changes. Polyacrylamide gel coverslips are useful for high resolution fluorescent and traction force microscopy and enable experiments involving single cell tracking. Modifications to the method can give rise to patterned surfaces and custom stiffness gradients (Tse and Engler, 2010). However, these protocols are not adaptable for routine cell culture or experiments that require large surface areas due to the inherently small sizes of the platforms. Additionally, they are not amenable to conditioned media generation for studying the release of secreted factors, RNA, extracellular vesicles, and other media components (Millar‐Haskell et al., 2022a). We focused on overcoming this size limitation to enable greater versatility in polyacrylamide gel platforms.
We have developed a method of synthesizing polyacrylamide gels inside glass dishes so that they can be used like normal tissue culture dishes (Figure 1). The process is much like casting a gel for polyacrylamide gel electrophoresis, except the gel is polymerized horizontally in a glass cell culture dish. The materials used in this protocol are easy to obtain, autoclave-safe, and re-usable. The polydimethylsiloxane (PDMS) ring and acrylic cover create a seal during polymerization, and the cover is removed after gelation. This format prevents the introduction of excess oxygen during polymerization and creates a flat surface for cell culture. Additionally, this protocol uses 145 mm glass dishes, but it can be adapted for any size, including traditionally used 100 mm glass dishes or, in cases where a custom shape is needed, by changing the dimensions of the PDMS ring. These dishes can be made in bulk and stored for later use as needed. We found that we could culture our cells (PANC-1 pancreatic cancer cells) in these dishes for up to a week with no loss of viability and minimal cell damage upon detaching them (Figure 2). Possible applications of this platform include conditioned media production, supernatant analysis (e.g., extracellular vesicle release or protein secretion), or large cell lysate collection for downstream analysis (Millar‐Haskell et al., 2022b).
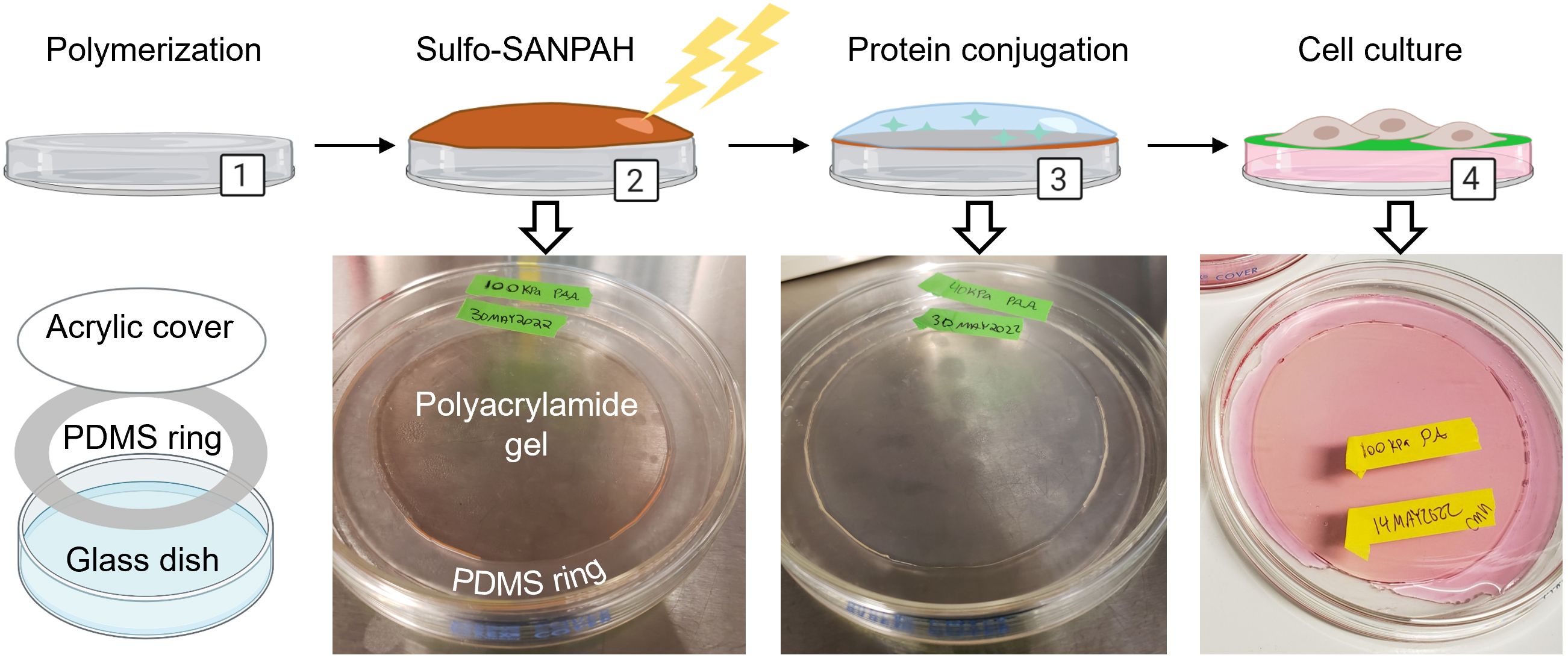
Figure 1. Overview for creating polyacrylamide dishes. (1) Polyacrylamide is synthesized in a glass dish using a PDMS ring and acrylic cover. (2) The crosslinker sulfo-SANPAH is activated on the polyacrylamide with UV light and the excess is rinsed off. (3) Extracellular matrix protein is diluted in buffer and incubated on the polyacrylamide to initiate conjugation. (4) After soaking dishes in HBS to remove unconjugated protein, dishes are pre-incubated with media and are ready for cell culture. Created with Biorender.com. Adapted from Millar-Haskell et al. (2022b).
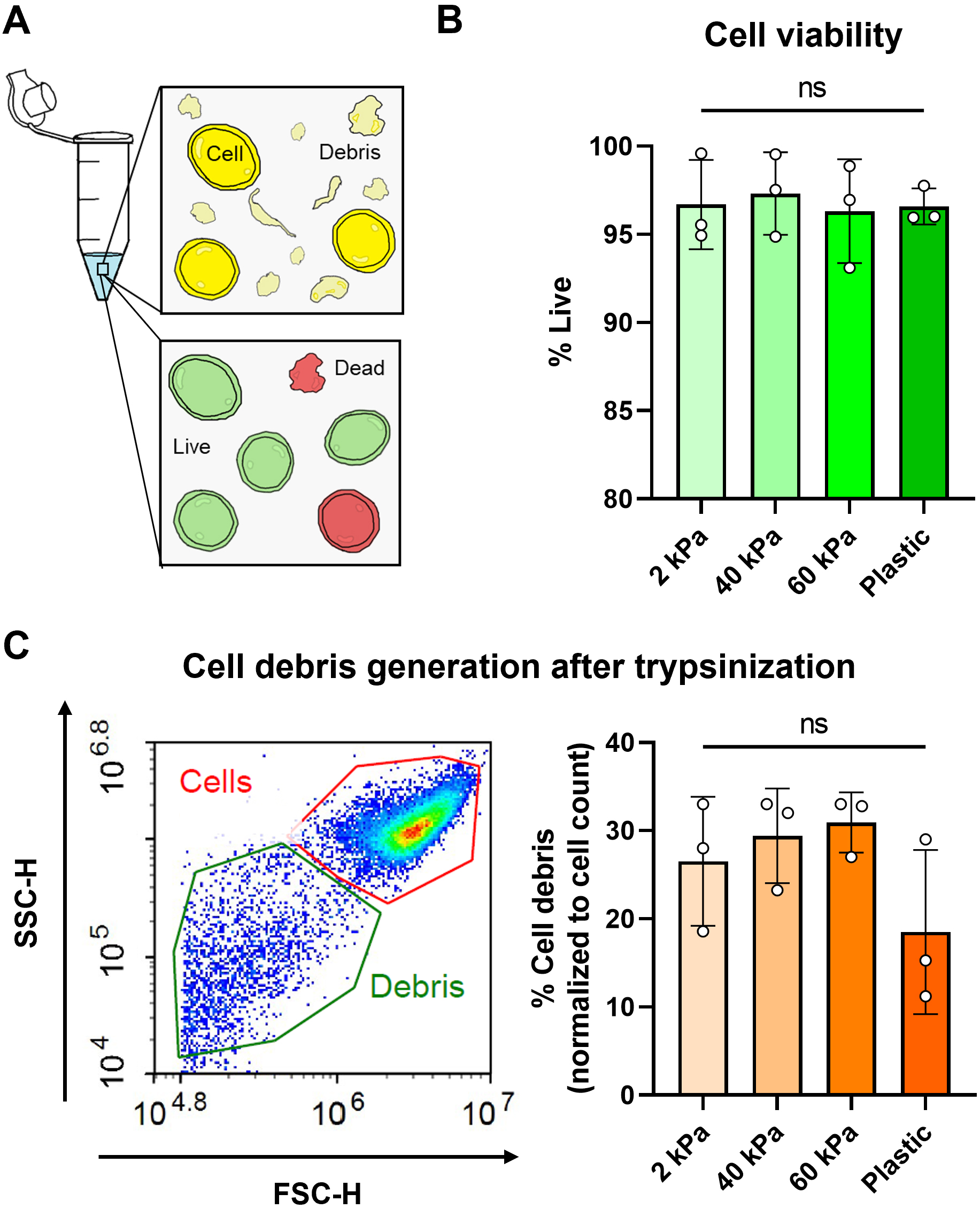
Figure 2. Cell viability assessment using calcein AM dye and flow cytometry. (A) Cartoon representation of cells in a tube after trypsinization with the presence of debris caused by cell death/compromised membranes. Cells were also stained using a live/dead viability assay. (B) Viability of PANC-1 cells cultured on polyacrylamide gel dishes of different elastic modulus and tissue culture plastic. Viability was quantified by percentage of calcein AM+ cells using flow cytometry. (C) Cell debris generation (which corresponds to cell damage and death) was normalized as a percentage of total cell count. Example plot with cell and debris gating is shown.
Materials and reagents
15 mL conical tubes (CELLTREAT, catalog number: 667016B)
Cleanroom wipes (Fisher Scientific, Contec, catalog number: 19-130-5932)
Plastic spoons and clear plastic cups (recommend Amazon or any major retailer)
Feather scalpel blades #11 (Electron Microscopy Sciences, catalog number: 72044-11)
Sylgard 184 silicone elastomer kit (Krayden, catalog number: DC4019862)
145 mm glass Petri dishes (VWR, catalog number: 25354-127)
145 mm plastic Petri dishes (VWR, catalog number: 82050-600)
0.2 μm PES bottle top filter (VWR, catalog number: 10040-436)
Autoclavable sterilization pouches (VWR, Cardinal Health, catalog number: 11213-033)
Sodium hydroxide (0.1 M NaOH)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
3-aminopropyltriethoxysilane (APTES) (Fisher Scientific, ACROS organics, catalog number: AC430941000); store at 4 °C, avoid contact with air
25% glutaraldehyde (GA) (VWR, Electron Microscopy Sciences, catalog number: 100504-788); store at 4 °C. We have found that this GA can be stored for up to three years without any changes to the end result
40% acrylamide (Sigma-Aldrich, catalog number: A4058); store at 4 °C
2% bis-acrylamide (Sigma-Aldrich, catalog number: M1533); store at 4 °C
Ammonium persulfate powder (APS) (VWR, catalog number: 97064-594), store at room temperature in a desiccator, strong oxidizer
Tetramethylethylenediamine (TEMED) (VWR, catalog number: 97064-684); flammable
125 mm clear acrylic disc (127 mm/5 inch acrylic discs may be found on Amazon, or acrylic sheets may be purchased from McMaster-Carr and laser cut into any size or shape)
Sulfo-SANPAH (Covachem, catalog number: 13414-100); store at 4 °C
HEPES (Sigma-Aldrich, catalog number: H3375-500G)
Protein of interest (e.g., collagen, Corning, catalog number: 354236); store at 4 °C
Hank’s buffered salt solution (HBSS) (ScienCell, catalog number: 0313)
Milli-Q water (or equivalent grade)
Isopropanol (70% solution)
Ethanol (70% solution)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: 276855)
Ammonium persulfate, 10% w/v solution (see Recipes)
Sulfo-SANPAH, 100 mg/mL solution (see Recipes)
HEPES-buffered saline (HBS) (see Recipes)
Equipment
Vacuum desiccator
Balance/weighing scale
Glass beaker (VWR, catalog number: 13912-284)
Filter forceps (VWR, catalog number: 89259-954)
Scalpel handle #3 (Fine Science Tools, catalog number: 10003-12)
Pipet aid and serological pipets (10, 25, 50 mL)
Set of micropipettes and tips (P10, P200, P1000)
Oven, set at 65 °C
Chemical hood
Biosafety cabinet with vacuum aspirator
Autoclave
UV lamp (e.g., 100-Watt 365 nm UVP lamp)
CO2humidified incubator, set at 37 °C
-20 °C freezer
4 °C refrigerator
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Millar-Haskell, C. S. and Gleghorn, J. P. (2023). A Large-format Polyacrylamide Gel with Controllable Matrix Mechanics for Mammalian Cell Culture and Conditioned Media Production. Bio-protocol 13(17): e4807. DOI: 10.21769/BioProtoc.4807.
分类
细胞生物学 > 细胞分离和培养 > 3D细胞培养
生物工程 > 生物医学工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












