- EN - English
- CN - 中文
Isolation of Embryonic Cardiomyocytes and Cell Proliferation Assay Using Genetically Engineered Reporter Mouse Model
使用基因工程报告小鼠模型分离胚胎心肌细胞并进行细胞增殖测定
发布: 2023年09月05日第13卷第17期 DOI: 10.21769/BioProtoc.4802 浏览次数: 1774
评审: Pilar Villacampa AlcubierreKyoung-Han KimMarina Sánchez PetidierChao WangAnonymous reviewer(s)
Abstract
Congenital heart disease (CHD) is often associated with myogenic defects. During heart development, cardiomyocyte growth requires essential cues from extrinsic factors such as insulin-like growth factor 2 (IGF-2). To determine whether and how growth factors account for embryonic cardiomyocyte proliferation, isolation followed by culturing of embryonic cardiomyocytes can be utilized as a useful tool for heart developmental studies. Current protocols for isolating cardiomyocytes from the heart do not include a cardiomyocyte-specific reporter to distinguish cardiomyocytes from other cell types. To optimize visualization of cardiomyocyte proliferation, our protocol utilizes a Tnnt2-promoter-driven H2B-GFP knock-in mouse model (TNNT2H2B-GFP/+) for in vitro visualization of nuclear-tagged cardiomyocyte-specific fluorescence. A cardiomyocyte-specific genetic reporter paired with an effective proliferation assay improves the reproducibility of mechanistic studies by increasing the accuracy of cell identification, proliferated cell counting, and cardiomyocyte tracking.
Key features
• This protocol refines previous methods of cardiomyocyte isolation to specifically target embryonic cardiomyocytes.
• UsesH2B-GFP/+cardiomyocyte reporters as identified by Yan et al. (2016).
• Traces cell proliferation with Phospho-Histone 3 (p-H3) assay.
• Has applications in assessing the role of growth factors in cardiomyocyte proliferation.
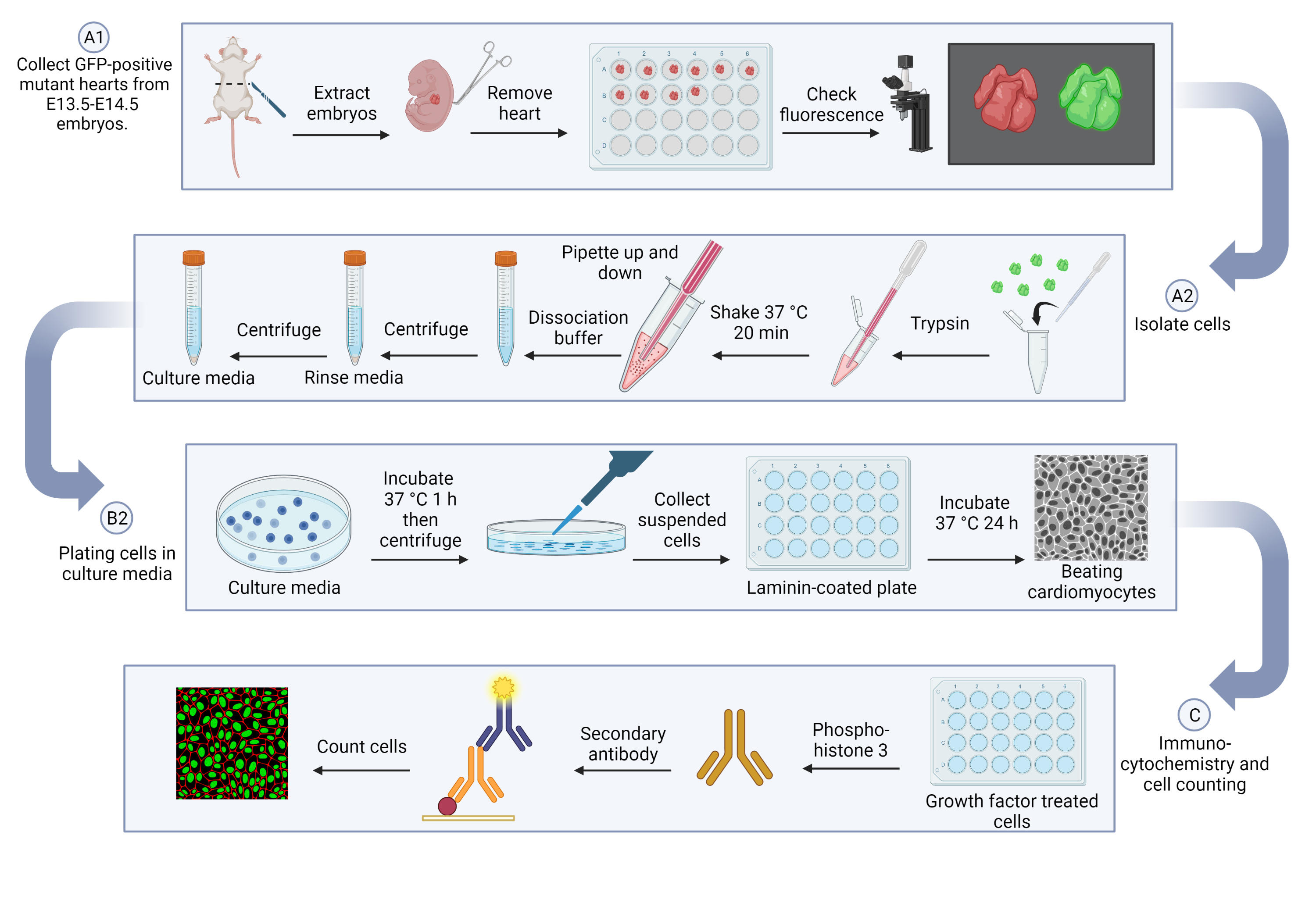
Graphical overview
Background
Myocardial expansion is one of the most critical cardiac developmental events. Failure of this process gives rise to many forms of congenital heart disease (CHD), which remains the most common cause of infant death (Zaidi and Brueckner, 2017). During heart development, cardiomyocytes intimately communicate with adjacent nonmyocytes, which promote cardiomyocyte proliferation through paracrine signals (Jang et al., 2022).
To study whether extrinsic cues contribute to embryonic cardiomyocyte proliferation, isolated cardiomyocytes from embryonic hearts can be used to explore the underlying mechanisms of myocardial growth. Here, a newly introduced genetically engineered reporter mouse model (Tnnt2H2B-GFP/+) (Yan et al., 2016) was paired with a proliferation assay. Since previous protocols have outlined the rudimentary trypsin digestion method for embryonic cardiomyocyte isolation (Rodgers et al., 2009), isolated cells were primarily cardiomyocytes but also included cardiac fibroblasts and cardiac endothelial cells. Thus, cardiomyocyte staining would be needed for further tracking of cardiomyocyte-specific proliferation within isolated cells. To monitor cardiomyocyte proliferation over time without the need of further staining, we used a cardiomyocyte specific reporter, cardiac Troponin T (Tnnt2), to definitively confirm cell type for accuracy in subsequent cell counting. With the inclusion of a proliferation assay, this protocol provides a reliable approach for in vitro assessments of growth factor effects on myocardial development.
This protocol consists of three key steps: embryonic cardiomyocyte isolation, cardiomyocyte culture, and immunostaining of cell proliferation markers. First, the hearts will be collected and digested with trypsin, and then further separated with dissociation buffer. For cardiomyocyte culture, cells will be pre-plated in culture media to remove other cell types, such as fibroblasts and endothelial cells, and will then be moved into a laminin-coated dish to support myocardial cells. To measure cell proliferation, serum-free media will be added to starve the cells and prevent residual growth factor influences; cells will then be treated with insulin-like growth factor 2 (IGF-2) to stimulate cardiomyocyte proliferation. Finally, immunocytochemistry staining with Phospho-Histone 3 (p-H3) will be applied for counting the proliferating cells as well as total GFP-positive cardiomyocytes.
Taken together, the proliferation assay with the genetic reporter mouse model will allow us to dissect the general mechanisms of the mitotic properties of cells, which can be applied to developmental research.
Materials and reagents
Biological materials
p-H3 (rabbit) (Ser10) antibody (Cell Signaling, catalog number: 9701S), store at -20 °C
Donkey anti-rabbit IgG (H+L), Alexa Fluor 555 (Life, catalog number: A31572)
100 ng/mL IGF-2 recombinant protein (R&D Systems, catalog number: 792-MG-050)
TNNT2H2B-GFP/+mice (from Dr. Chen-Leng Cai, Background Strain: C57BL/6J)
Reagents
1× PBS pH 7.4 (Gibco, catalog number: 10010-023)
1 M HEPES (Gibco, catalog number: 15630-060), store at 4 °C
0.5 M EDTA pH 8.0 (Gibco, UltraPure, catalog number: 15575), store at 4 °C
1:250 Trypsin (Gibco, catalog number: 27250-018)
HBSS solution (Gibco, catalog number: 14175-095)
Horse serum (Gibco, catalog number: 16050-114), store at -80 °C
FBS (Gibco, catalog number: 10437-028), store at -80 °C
100× antibiotic-antimycotic (Gibco, catalog number: 15240-062)
1× DMEM [-] Ca2+(Gibco, catalog number: 21068-028), store at 4 °C
Laminin (Corning, catalog number: 354232), store at -80 °C
1× DPBS (Ca2+/Mg2+) (Corning, catalog number: 21-030-CV)
1× Opti-MEM (Gibco, catalog number: 31985-070), store at 4 °C
IC fixation buffer (Invitrogen, catalog number: 00822249), store at 4 °C
Triton X-100 (Fisher Biotech, catalog number: BP151-500)
Donkey serum (Fisher Brand, catalog number: 03-395-464)
0.1 μg/mL DAPI (1:1,000 working concentration) (Sigma, catalog number: D9542)
1× DMEM [+] Ca2+(Gibco, catalog number: 11965-092), store at 4 °C
Solutions
Trypsin solution (see Recipes)
Dissociation buffer (see Recipes)
Rinse media (see Recipes)
Culture media (see Recipes)
Recipes
Trypsin solution (in 1× HBSS)
Reagent Final concentration Quantity (25 mL) HEPES (1 M) 10 mM 250 μL EDTA (0.5 M) 0.54 mM 27 μL Trypsin 0.25% 0.0625 g HBSS solution (1×) n/a 25 mL Dissociation buffer (in 1× HBSS) at 4 °C
Reagent Final concentration Quantity (25 mL) Horse serum 10% 2.5 mL FBS 5% 1.25 mL HEPES (1 M)
HBSS Solution (1×)
n/a
n/a
250 μL
21.2 mL
Rinse media (in Ca2+free DMEM)
Reagent Final concentration Quantity (50 mL) Horse serum 10% 5 mL FBS 5% 2.5 mL Antibiotic-antimycotic
Ca2+-free DMEM
1%
n/a
0.5 mL
42 mL
Culture media (in Opti-MEM)
Reagent Final concentration Quantity (20 mL) Horse serum 10% 2 mL FBS 5% 1 mL Antibiotic-antimycotic
Opti-MEM
0.1%
n/a
20 μL
17 mL
Note: In isolating and plating the cardiomyocyte step, high quality and optimized concentration of FBS and horse serum are critical for cell viability and cell adherence.
Laboratory supplies
Surgical scissors (Roboz, catalog number: RS-5882)
Curved forceps (Stoelting, catalog number: 52102-54P)
7 mL transfer pipette (Globe Scientific, catalog number: 135038)
24-well culture plate (Denville, catalog number: T1024)
1.5 mL micro-centrifuge tube (Stellar Scientific, T3 Tube, catalog number: T17-300)
60 mm cell culture dish (Eppendorf, catalog number: 0030701111)
70 μm mesh cell strainer (Swish, catalog number: TC70-MT-3)
15 mL centrifuge tube (Denville, Thomas Scientific, catalog number: 1158R11)
Equipment
M205 FCA microscope (Leica)
Hybridization oven/shaker (Amersham Biosciences)
Centrifuge 5425 R (Eppendorf)
TSX60086A -80 °C freezer (Thermo Scientific)
Isotemp CO2incubator (Fisher brand)
Centrifuge 5702 (Eppendorf)
Handheld automated cell counter (Millipore, Scepter 3.0, catalog number: PHCC30000)
60 μm sensors (Millipore, Scepter 3.0, PHCC360050)
-20 °C freezer (Fisher Scientific, Isotemp)
4 °C fridge (Insignia)
BZ-X800 microscope (Keyence)
Standard orbital shaker, Model 5000 (VWR, catalog number: 89032-100)
Software and datasets
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Beall, M., Li, D. and Jang, J. (2023). Isolation of Embryonic Cardiomyocytes and Cell Proliferation Assay Using Genetically Engineered Reporter Mouse Model. Bio-protocol 13(17): e4802. DOI: 10.21769/BioProtoc.4802.
分类
发育生物学 > 细胞生长和命运决定 > 增殖
细胞生物学 > 细胞分离和培养 > 单层培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link