- EN - English
- CN - 中文
Methods to Quantify the Dynamic Recycling of Plasma Membrane Channels
质膜通道动态循环的量化方法
发布: 2023年09月05日第13卷第17期 DOI: 10.21769/BioProtoc.4800 浏览次数: 1668
评审: Emilia KrypotouXin XuAnonymous reviewer(s)

相关实验方案
用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2283 阅读
Abstract
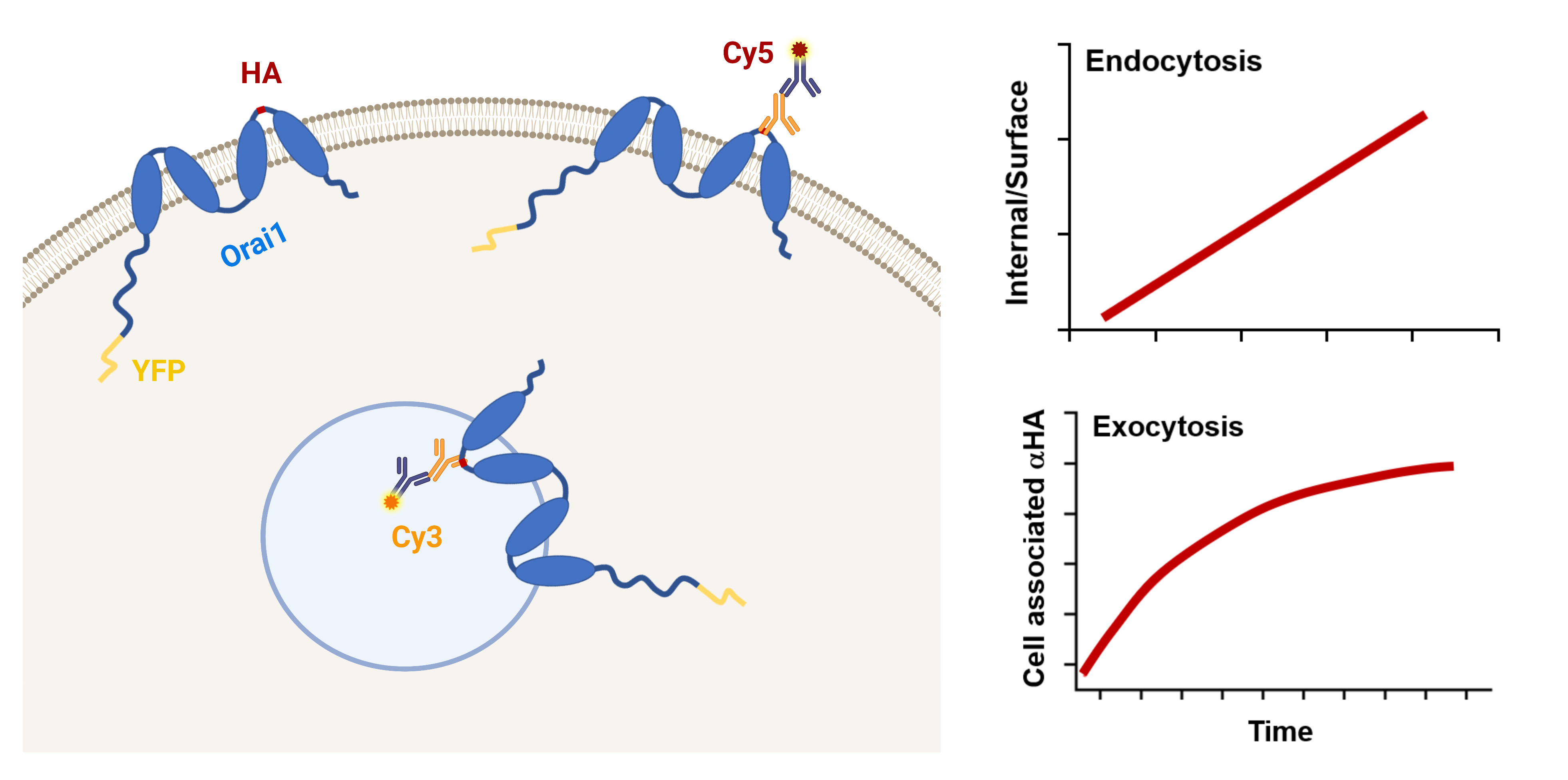
Store-operated Ca2+ entry (SOCE) is a ubiquitous Ca2+ signaling modality mediated by Orai Ca2+ channels at the plasma membrane (PM) and the endoplasmic reticulum (ER) Ca2+ sensors STIM1/2. At steady state, Orai1 constitutively cycles between an intracellular compartment and the PM. Orai1 PM residency is modulated by its endocytosis and exocytosis rates. Therefore, Orai1 trafficking represents an important regulatory mechanism to define the levels of Ca2+ influx. Here, we present a protocol using the dually tagged YFP-HA-Orai1 with a cytosolic YFP and extracellular hemagglutinin (HA) tag to quantify Orai1 cycling rates. For measuring Orai1 endocytosis, cells expressing YFP-HA-Orai1 are incubated with mouse anti-HA antibody for various periods of time before being fixed and stained for surface Orai1 with Cy5-labeled anti-mouse IgG. The cells are fixed again, permeabilized, and stained with Cy3-labeled anti-mouse IgG to reveal anti-HA that has been internalized. To quantify Orai1 exocytosis rate, cells are incubated with anti-HA antibody for various incubation periods before being fixed, permeabilized, and then stained with Cy5-labeled anti-mouse IgG. The Cy5/YFP ratio is plotted over time and fitted with a mono-exponential growth curve to determine exocytosis rate. Although the described assays were developed to measure Orai1 trafficking, they are readily adaptable to other PM channels.
Key features
• Detailed protocols to quantify endocytosis and exocytosis rates of Orai1 at the plasma membrane that can be used in various cell lines.
• The endocytosis and exocytosis assays are readily adaptable to study the trafficking of other plasma membrane channels.
Graphical overview
Background
Orai1 is the pore-forming subunit of the store-operated Ca2+ entry (SOCE) channel. It mediates Ca2+ influx into the cell following direct coupling with ER Ca2+ sensor STIM1, in response to store depletion following Ca2+ release from stores (Luik et al., 2006; Prakriya et al., 2006; Vig et al., 2006; Yeromin et al., 2006). SOCE is central to various physiological processes, and its dysfunction is associated with serious pathologies in humans (Feske, 2011, Feske et al., 2015; Gruszczynska-Biegala et al., 2021; Yu et al., 2021). A key feature in SOCE regulation is the residence of Orai1 at the plasma membrane (PM) and the amount of reserve Orai1 in intracellular compartments that can potentially contribute to the PM pool following SOCE activation.
The steady-state amount of Orai1 at the PM is determined by the rates of endocytosis and exocytosis. Alterations in these rates modulate Orai1 PM levels. Several protocols have been used to quantify the amount of surface and intracellular Orai1, including imaging of fluorescently tagged Orai1 and biotinylation (Yu et al., 2009; Cox et al., 2013; Yeh et al., 2020; Kim et al., 2021; Wrennall et al., 2022). These methodologies provide crude estimates of Orai1 distribution, but they lack the sensitivity and time resolution to determine Orai1 recycling kinetics at the PM. Herein, we describe protocols to quantify the kinetics of Orai1 trafficking using a dually tagged human Orai1 (YFP-HA-Orai1) with a cytosolic YFP at the N-terminus and a hemagglutinin (HA) tag inserted in the second extracellular loop (Park et al., 2009; Hodeify et al., 2015 and 2018). This dual reporter allows quantification of total Orai1 using YFP fluorescence and concomitantly follows its trafficking using the HA-antibody at the single-cell level. The PM and intracellular pools can be differentially labeled by staining unpermeabilized and permeabilized cells with different secondary antibodies (Cy5- or Cy3-labeled). Combining these approaches with time courses of anti-HA antibody feeding allows one to carefully quantify Orai1 endocytosis and exocytosis rates. The presented protocols can be easily adapted to study recycling dynamics of PM channels in a wide range of mammalian cells.
Materials and reagents
Biological materials
TRVb-1 cells, a CHO cell line lacking the endogenous transferrin receptor and stably expressing the human transferrin receptor (McGraw et al., 1987). Any readily transfectable cell line can be substituted for CHO cells
Materials
Plasmid pDS-YFP-HA-Orai1 encoding a dually tagged Orai1 with YFP at the N-terminus and an HA tag inserted in the second extracellular loop (see Hodeify et al., 2015)
Bicarbonate-buffered Ham’s F-12 medium (Invitrogen, catalog number: 11765054)
Cy3- and Cy5-conjugated anti-mouse secondary antibodies (Invitrogen, catalog numbers: A10521 and A10524)
Purified monoclonal anti-HA.11 Epitope Tag antibody (Covance, catalog number: MMS-101P)
Poly-D-lysine coated glass-bottomed plates (MatTek Corporation, catalog number: P35GC-1.5-14-C)
Lipofectamine 2000 (Invitrogen, catalog number: 11668019)
GibcoTM Opti-MEMTM I reduced serum medium, 500 mL (Thermo Fisher Scientific, catalog number: 31985047)
Dulbecco’s phosphate buffered saline (PBS) (Sigma, catalog number: D8537-500ML)
Penicillin-streptomycin (GIBCO REF, catalog number: 15140122)
Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 16005-1KG-R)
Triton X-100 (Sigma, catalog number: T8787-50ML)
10% heat-inactivated FBS (Sigma, catalog number: F4135-500ML)
FalconTM 15 mL conical centrifuge tubes, polypropylene
FalconTM conical centrifuge tubes, 15 mL (Corning, catalog number: 352095)
Microcentrifuge tubes, 1.5 mL (Sigma-Aldrich, catalog number: HS4323)
250 mL glass Erlenmeyer flask (VWR, catalog number: 89000-362-250ML)
Solutions
Paraformaldehyde, 4% in PBS (see Recipes)
PBS containing 0.1% (w/v) Triton X-100 (see Recipes)
Recipes
Paraformaldehyde, 4% in PBS
Add 80 mL of 1× PBS into a 250 mL glass Erlenmeyer flask with a clean magnetic stir bar.
Heat the solution while stirring to approximately 60 and add 4 g of PFA powder (powder will not immediately dissolve).
Slowly raise the pH by adding 1 N NaOH dropwise until the solution clears.
Remove from heat and allow to cool to room temperature (RT).
Adjust the volume to 100 mL with 1× PBS.
Filter through aqueous filter paper to remove undissolved particles.
PBS containing 0.1% (w/v) Triton X-100
Add 5 μL of Triton X-100 to 50 mL of 1× PBS.
Equipment
Leica SP inverted confocal imaging microscope (Leica; Lasertechnik; S/N: 5100001464)
Magnetic stirrer with hotplate (IKA magnetic stirrer RCT basic, Ident. No.: 0025006052)
Cell culture incubator model: Sanyo MIR-262 Lab Incubator 153 L Capacity w/ Two Shelves S/N: 08020051 (Sanyo)
Software
ImageJ software (http://imagej.net/mbf/)
Adobe Photoshop CS3
Origin (v 2019b)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Hodeify, R. and Machaca, K. (2023). Methods to Quantify the Dynamic Recycling of Plasma Membrane Channels. Bio-protocol 13(17): e4800. DOI: 10.21769/BioProtoc.4800.
- Hodeify, R., Nandakumar, M., Own, M., Courjaret, R. J., Graumann, J., Hubrack, S. Z. and Machaca, K. (2018). The CCT chaperonin is a novel regulator of Ca2+ signaling through modulation of Orai1 trafficking. Sci. Adv. 4(9): eaau1935.
分类
细胞生物学 > 基于细胞的分析方法 > 内吞作用
细胞生物学 > 细胞成像 > 荧光
细胞生物学 > 细胞信号传导 > 胞内信号传导
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link