- EN - English
- CN - 中文
The Effects of Whole-body Cold-water Immersion on Brain Connectivity Related to the Affective State in Adults Using fMRI: A Protocol of a Pre-post Experimental Design
使用 fMRI 进行全身冷水浸泡对与成人情感状态相关的大脑连接的影响:前后实验设计方案
(*contributed equally to this work) 发布: 2023年09月05日第13卷第17期 DOI: 10.21769/BioProtoc.4794 浏览次数: 2622
评审: Oneil Girish BhalalaAnonymous reviewer(s)
Abstract
An emerging body of behavioural studies indicates that regular swimming in cold water has positive effects on mental health and wellbeing, such as reducing fatigue, improving mood, and lessening depressive symptoms. Moreover, some studies reported immediate effects of cold-water immersion (CWI) on elevating mood and increasing a positive emotional state. However, the neural mechanisms underlying these effects remain largely unknown. The lack of studies using neuroimaging techniques to investigate how a whole-body CWI affects neural processes has partly resulted from the lack of a tested experimental protocol. Previous protocols administered tonic limb cooling (1–10 °C) while recording functional magnetic resonance (fMRI) signals. However, using very low water temperature constitutes points of contrast to painful experiences that are different from what we experience after a whole-body head-out CWI. In our protocol, healthy adults unhabituated to cold water were scanned twice: immediately before (pre-CWI) and after (post-CWI) immersion in cold water (water temperature 20 °C) for 5 min. We recorded cardiac and ventilatory responses to CWI and assessed self-reported changes in positive and negative affects. Our protocol showed reliable changes in brain connectivity after a short exposure to cold water, thus enabling its use as a tested experimental framework in future studies.
Graphical overview
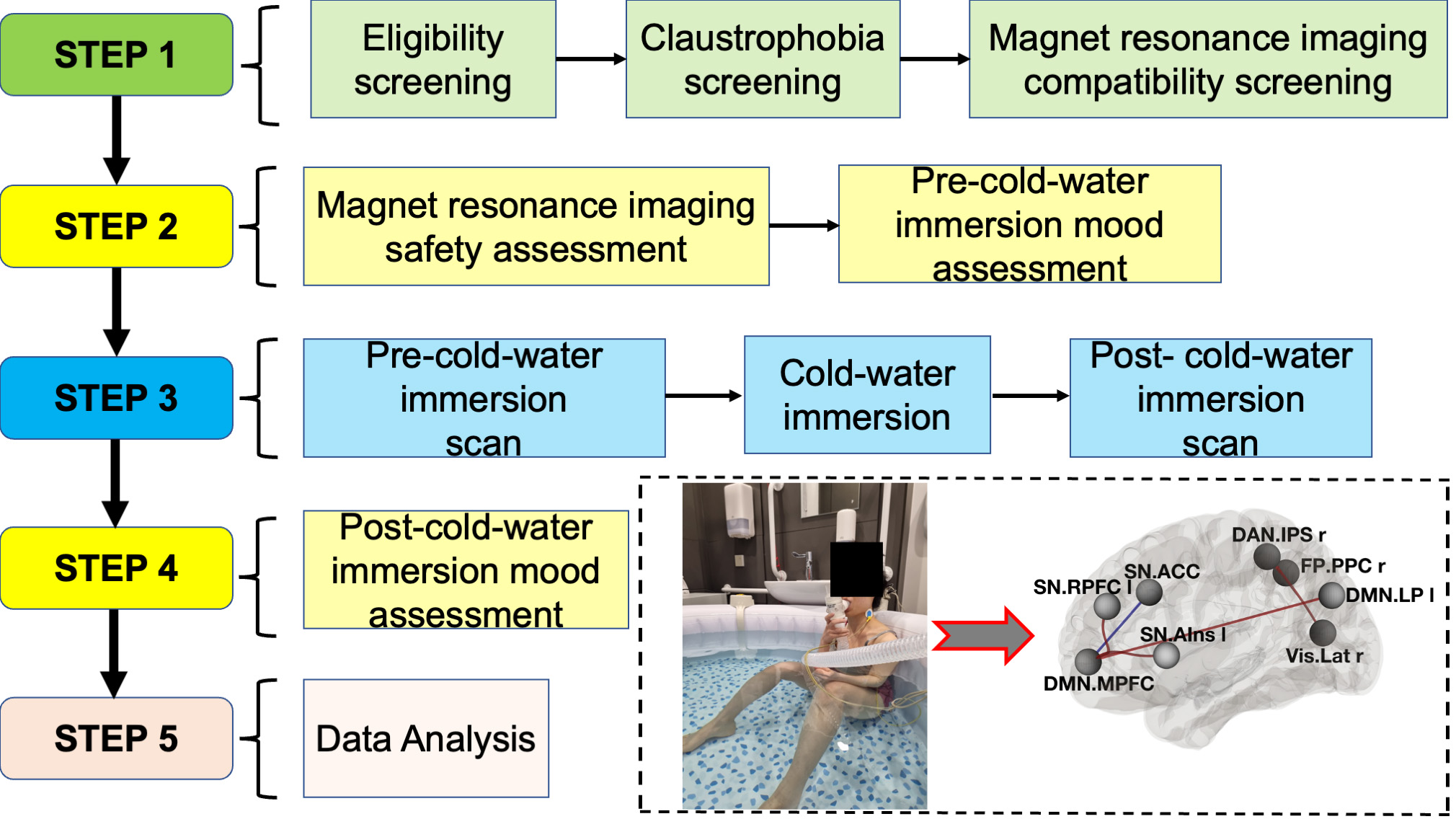
Background
Cold-water swimming has become increasingly popular (Griffiths and Turner, 2022). With much evidence suggesting this activity has risks (Tipton, 1989; Tipton et al., 2017; Knechtle et al., 2020), the beneficial effects of cold-water immersion (CWI) have been linked to enhancing a positive mood state (Massey et al., 2022), higher perceived psycho-physical wellbeing (Huttunen, et al., 2004; Demori et al., 2021), and ameliorating mood disorders (Lindeman et al., 2002). It has been suggested that whole-body exposure to cold triggers a release of neurotransmitters such as serotonin, cortisol, dopamine, norepinephrine, and β-endorphin (Hirvonen et al., 2002), which play a crucial role in emotion (Gu et al., 2016; Canli and Lesch, 2007) and stress regulation (Godoy et al., 2018). Furthermore, emerging evidence indicates that the therapeutic benefits of cold water, such as increasing positive and decreasing negative affects, may be gained even from a single exposure (Massey et al., 2020; Kelly and Bird, 2022). This evidence was derived from behavioural studies using a questionnaire design and, as such, the neural mechanisms of the effects of CWI on affective processes remain largely unknown.
Only a few studies investigating how CWI changes brain activity have been conducted in humans (Jarrahi et al., 2017; Bitar et al., 2020; Grouper et al., 2022). However, these studies focused on assessing brain activity and its association to cognitive-affective aspects of pain, by using an experimental protocol of administered tonic limb cooling. It must be noted that physiological responses to limb cooling using very low water temperatures (1–10 °C) constitute points of contrast that result in painful experiences, which are different from what we experience after taking a cold shower or swimming in higher temperature open waters.
We developed and tested a novel protocol to examine the effects of short-term, head-out, whole-body CWI on changes in brain functional connectivity. This protocol can be adapted for other research questions focusing on brain-behaviour interactions [e.g., linking personality traits, cognitive functions such as decision making, memory, and perception to the brain's functional architecture (Cole et al., 2014)]. A tight control of experimental factors in our protocol makes it easy to replicate and minimise confounding effects.
Materials and reagents
Non-effervescent Haz-Tab (4.5 g NADDC per tablet) (Guest Medical, catalog number: H8801) made up to 1,000 ppm (one tablet per 2.5 L)
The Positive and Negative Affect Schedule (PANAS) (Watson et al., 1988)
Appropriately sized hospital scrubs for each participant
Blankets
Equipment
MRI machine (3-Tesla Siemens Magnetom Lumina, 32-channels head-coil)
Water heater (Laz-y-spa, UK)
Water pump (Laz-y-spa, UK)
Water chiller unit (Grant Instruments, UK)
Three-lead electrocardiogram (ECG, Fukuda Denshi, UK)
ECG electrodes (Ambu® Blue Sensor P, Malaysia)
Electrodes covered with Tegaderm dressings (3M, Germany)
35 mm respiratory hose (Cranlea, UK)
Reusable nose clip (Cranlea, UK)
Two-way T-shape non-rebreathing valve (Hans Rudolf, US)
Respiratory turbine (K.L. Engineering, US)
Thermistor pod (ML309, AD Instruments, Australia)
Skin temperature thermistor (MLT422/A, Australia)
Digital recorder (Powerlab, AD Instruments, Australia)
Laptop connected to the Powerlab and programmed to a sampling rate of 400 Hz (Chart software, AD Instruments Australia)
Scanning parameters
Anatomical scan: T1-weighted MPRAGE images with the following parameters: repetition time (TR) = 1,900 ms, echo time (TE) = 2.74 ms, flip angle = 8°, field of view (FOV) = 256 × 256 mm2, voxel size = 1.0 × 1.0 × 1.0 mm3, and 192 axial slices.
Functional scan: blood oxygenation level–dependent (BOLD) contrast whole-brain functional images were acquired using a T2-weighted gradient-echo Echo Planar Imaging (EPI) sequence with a 32-channel head coil. Sequence duration 13.12 min. Acquisition parameters: TR = 2,680 ms; TE = 30 ms; matrix size = 64 × 64 mm2; slice thickness = 2.5 mm, spacing between slices = 2.5 mm, flip angle = 80°; 292 volumes with 48 axial slices were measured in interleaved slice order and positioned along a line to the anterior-posterior commissure (AC-PC orientation). An automated high-order shimming technique was used to maximise magnetic field homogeneity.
Software
MATLAB 2022a (MATLAB and Statistics Toolbox Release 2012b, The MathWorks, Natick, Massachusetts, United States)
CONN (v.2021a): A functional connectivity toolbox for correlated and anticorrelated brain networks (Whitfield-Gabrieli and Nieto-Castanon, 2012, http://www.nitrc.org/projects/conn)
JASP (JASP Team, 2022, version 0.16.3) (Computer software)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Yankouskaya, A., Massey, H., Totman, J. J., Lai, L. H. and Williamson, R. (2023). The Effects of Whole-body Cold-water Immersion on Brain Connectivity Related to the Affective State in Adults Using fMRI: A Protocol of a Pre-post Experimental Design. Bio-protocol 13(17): e4794. DOI: 10.21769/BioProtoc.4794.
分类
神经科学 > 行为神经科学
神经科学 > 基础技术 > 功能性磁共振成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











