- EN - English
- CN - 中文
Binging from Food to Alcohol: A Sequential Interaction Between Binging Behaviors in Male Wistar Rats
从食物到酒精的狂饮:雄性Wistar大鼠嗜酒行为之间的顺序相互作用
(*contributed equally to this work) 发布: 2023年08月05日第13卷第15期 DOI: 10.21769/BioProtoc.4781 浏览次数: 1345
评审: Alejandro GrauMohammed Mostafizur RahmanAnonymous reviewer(s)
Abstract
The development of excessive alcohol (ethanol) and/or highly palatable food self-administration is an essential task to elucidate the neurobiological mechanisms that underlie these behaviors. Previous work has highlighted that ethanol self-administration is modulated by both the induction of aversive states (i.e., stress or frustration) and by the concurrent availability of appetitive stimuli (e.g., food). In our protocol, rats are food deprived for three days until they reach 82%–85% of their ad libitum weight. After that, rats are exposed daily for 10 days to a brief binge or control eating experience with highly sugary and palatable food (i.e., the ingestion of 11.66 and 0.97 kcal/3 min, respectively), which is followed by a two-bottle-choice test (ethanol vs. water) in their home cages for 90 min. This model induces robust binge eating, which is followed by a selective increase in ethanol self-administration. Therefore, this protocol allows to study: a) behavioral and neurobiological factors related to binge eating, b) different stages of alcohol use, and c) interactions between the latter and other addictive-like behaviors, like binge eating.
Keywords: Alcohol (酒精)Background
Animal models of binge drinking (BD) are crucial for clarifying the neurobiological and psychobiological mechanisms underlying this harmful behavior [see Jeanblanc et al. (2019) for a review]. Considerable advances have been made by forcibly administering ethanol (Becker and Lopez, 2016), employing operant paradigms (Simms et al., 2010), developing strains with a genetic predisposition to ethanol (Colombo et al., 2014), or using self-administration models in Wistar rats where ethanol is presented in an intermittent and temporally restricted fashion (Salguero et al, 2020; Ruiz-Leyva et al, 2020). These animal models, however, do not always mimic the contextual variables surrounding human drinking behaviors or fail to achieve pharmacologically relevant blood ethanol concentrations within the proposed time frame for this type of consumption. Moreover, alcohol use is driven by several factors. For instance, it has been observed that the induction of aversive states in conjunction with ethanol availability generally leads to an increase in ethanol consumption and favors the development of psychiatric diseases—including alcohol use disorders (AUD)—via epigenetic changes [for a review, see Pucci et al. (2019)]. In addition, the induction of frustration and anxiety (Díaz-Morán et al., 2013a and 2013b; Sabariego et al, 2013; Kawasaki et al., 2017; Jiménez-García et al., 2019) is associated with increases in ethanol consumption as a coping strategy (Ramirez-Castillo et al., 2019). Paradoxically, exposure to appetitive stimuli (i.e., food) also enhances ethanol consumption. Seminal studies showed that concurrent access to food and ethanol enhanced drinking behaviors in animals (Mello and Mendelson, 1971; Meisch and Thompson, 1972). In this respect, both substance use disorders (including AUD) and BD appear to be comorbid (or at least closely related) with binge eating (BE) (Ferriter and Ray; 2011; Munn-Chernoff et al., 2020). Conceptually, BE involves ingestion of large amounts of palatable (sugary and/or fatty) food in a short period of time, associated with a feeling of loss of control [see Dingemans et al. (2017) for a review].
The present protocol aims to serve as a guide for researchers interested in the study of the interaction of binge-like behaviors and should help replicability efforts. It is also an improvement over the models described above. An exhaustive description of the novel animal model of BE-BD interaction originally developed by Ruiz-Leyva et al. (2022) is presented. Here, the self-administration model induced remarkably high levels of absolute ethanol consumption (approximately 5 g/kg/90 min) and ethanol preference (80%–90%), much greater than those reported by other preclinical models. For instance, rats exposed to intermittent access to 20% ethanol in a two-bottle-choice procedure achieved 9–10 g/kg, yet they did that in a 24 h period, whereas those tested in the drinking-in-the-dark-multiple-scheduled access rarely exceed 5–6.5 g/kg/day. In this model, food-deprived rats are briefly exposed to a measurable amount of highly palatable sugary pellets immediately followed by a two-bottle-choice test (ethanol vs. water). Here, the authors reported dramatic and specific increases in ethanol intake as the BE behavior develops, leading to pharmacologically relevant blood ethanol concentrations. In addition, ethanol consumption turned compulsive by the last sessions and sensitive to naltrexone administration.
Materials and reagents
Anti-drip bottles with capacity of 150 mL (Classic Drinker de Luxe; Zooplus, Munich, Germany)
Filtered and autoclaved tap water
Rodent Dustless precision pellets (DPPs) (45 mg each, nutritional profile: 59.1% carbohydrate, 18.7% protein, 5.6% fat, 3.6 kcal/g) (Bio-serv, catalog number: F0021). Shelf life: 12 months. Store in a dry and ventilated place. The container should be tightly closed and protected from moisture
Ethyl alcohol (ethanol 96%, v/v; CH3CH2OH; 46.07 g/mol) (PanReac AppliChem, catalog number: 141085). This substance is highly flammable (liquid or vapor) and causes severe eye irritation. Store in a cool, ventilated place away from heat sources such as hot surfaces
Animals: adult male Wistar HAN rats (Envigo Laboratories, Barcelona, Spain), aged 70–80 days and weighing 240 g (± 37) at the beginning of the procedure
Equipment
Rectangular polycarbonate cages (42.5 cm × 26.5 cm × 15 cm) (Figure 1A)
Timer and chronometer (Digital Onstart 100) (Figure 1B)
Small plastic cups for depositing the DPPs
Weighing scale (WLC 1/A2 Precision Balance; Radwag©) (Figure 1C)
Plastic syringes (15 mL) and crystal flasks (600 mL) for diluting ethanol solutions (Figure 1D)
Personal protective equipment (laboratory coat, gloves, masks, etc.)
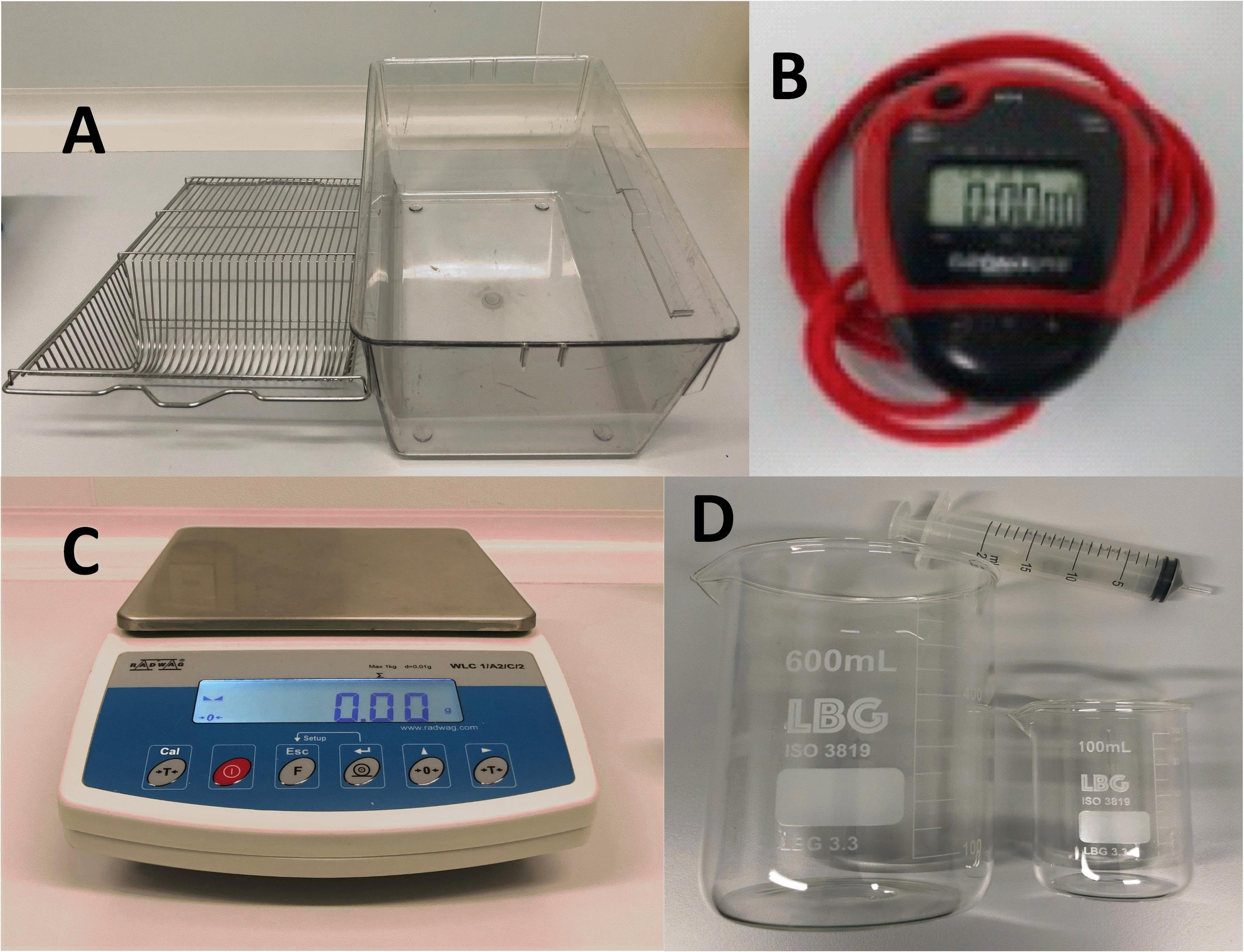
Figure 1. Equipment. A. Rectangular polycarbonate cage with grid. B. Timer and chronometer. C. Weighing. D. Syringe and flasks.
The alcohol concentrations of 6% or 10% were achieved by pouring 470 (or 450) mL of filtered and autoclaved tap water into 500 mL flasks and then adding 30 (or 50) mL of alcohol with the plastic syringe. Next, the solution was gently stirred to ensure proper dissolution of the alcohol.
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Cuesta-Martínez, S., Ruiz-Leyva, L., Jiménez-García, A. M., Aparicio-Mescua, T., López-Guarnido, O., Pautassi, R. M., Morón, I. and Cendán, C. M. (2023). Binging from Food to Alcohol: A Sequential Interaction Between Binging Behaviors in Male Wistar Rats. Bio-protocol 13(15): e4781. DOI: 10.21769/BioProtoc.4781.
分类
神经科学 > 行为神经科学 > 实验动物模型
神经科学 > 行为神经科学 > 成瘾
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












