- EN - English
- CN - 中文
Production and Purification of Cell Culture–generated Hepatitis B Virus by Transient Transfection and Density Gradient
通过瞬时转染和密度梯度法生产和纯化细胞培养产生的乙型肝炎病毒
发布: 2023年07月20日第13卷第14期 DOI: 10.21769/BioProtoc.4779 浏览次数: 1903
评审: Saskia F. ErttmannAnonymous reviewer(s)

相关实验方案

诱导型HIV-1库削减检测(HIVRRA):用于评估外周血单个核细胞中HIV-1潜伏库清除策略毒性与效力的快速敏感方法
Jade Jansen [...] Neeltje A. Kootstra
2025年07月20日 2460 阅读
Abstract
An efficient cell culture system for hepatitis B virus (HBV) is indispensable for research on viral characteristics and antiviral agents. Currently, for HBV infection assays in cell culture, HBV genome-integrated cell line–derived viruses are commonly used. However, these viruses are not suitable for the evaluation of polymorphism-dependent viral characteristics or resistant mutations against anti-viral agents. To detect the infection of cell culture–generated HBV (HBVcc) by the transient transfection of the HBV molecular clone, a large amount of purified viruses is needed, because such viruses exhibit limited infection efficiencies in cell culture. Here, we describe how to generate and purify HBVcc by the transient transfection of HBV molecular clones. This system provides a powerful tool for studying the infection and propagation of HBV and for developing anti-viral agents against HBV.
Keywords: HBV (HBV)Background
Hepatitis B virus (HBV) infection is a significant cause of chronic liver disease, including cirrhosis and hepatocellular carcinoma. Although effective vaccines against HBV infection are available in many countries, the global prevalence of HBV is estimated to be over 290 million. The eradication of chronic HBV infection by the current treatment strategy is not expected, because HBV covalently closed circular DNA in hepatocytes cannot be eliminated. To explore novel anti-HBV agents, a system for the infection and replication of HBV in cell culture is indispensable. Sodium taurocholate cotransporting polypeptide (NTCP) was identified as an HBV receptor, and NTCP-transduced HepG2 or HuH-7 cells contributed to the observation of HBV infection and replication in cell culture (König et al., 2019; Otoguro et al., 2020). In such cell culture systems for HBV, viruses derived from HBV genotype D genome-integrated cell lines, such as HepG2.2.15 or HepAD-38, are used. However, such viruses are not suitable for investigating the effects of strain-specific characteristics or resistance-related polymorphisms on the effectiveness of anti-HBV agents. For these purposes, the viruses obtained by the transient transfection of the HBV molecular clone are used. However, these viruses have limited infection efficiencies in cell culture. Therefore, methods for the efficient production of cell culture–generated HBV (HBVcc) and for the purification of infectious viruses will be important (Washizaki et al., 2022).
Materials and reagents
Production of HBVcc
HBV molecular clone plasmid
A replication-competent HBV molecular clone with a 1.38-fold genome length of HBV genotype C strain (accession number: AB246345) (Figure 1) (Murayama et al., 2021) inserted into the pUC19 plasmid is prepared. This clone is introduced with a precore stop mutation (G1896A) to evaluate the production of HBc proteins by measuring the HBcrAg level excluding HBeAg. HBeAg is not produced from this construct.
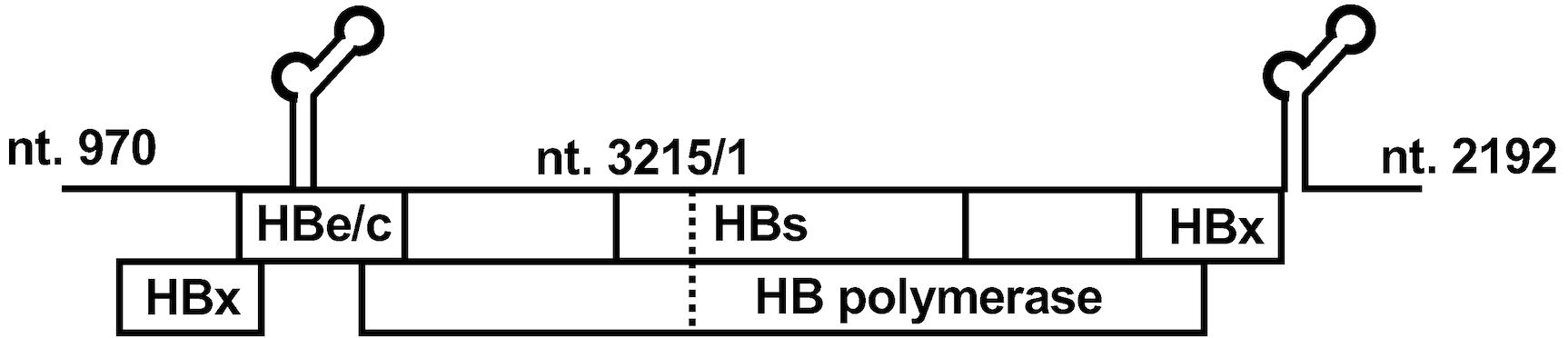
Figure 1. Structure of the hepatitis B virus (HBV) molecular clone for cell culture-generated HBV (HBVcc). Structure of the plasmid of the HBV molecular clone. This plasmid contains a 1.38-fold HBV genome (4,438 bp) of the HBV genotype C strain. This construct generates pregenomic RNA and expresses all HBV proteins.Sodium taurocholate cotransporting polypeptide (NTCP)-transduced cells
HepG2-NTCPsec+ (provided by Dr. Marc Peter Windisch; Institut Pasteur Korea, Seoul, South Korea) (König et al., 2019). These cells were cultured with HepG2-NTCPsec+ culture medium (see Recipes) supplemented with Blasticidin (5µ g/mL)
Dulbecco’s modified Eagle medium (DMEM) (FUJIFILM Wako Pure Chemical Corporation, catalog number: 044-29765)
Fetal bovine serum (FBS) (Sigma-Aldrich, catalog number: F7524)
MEM non-essential amino acids solution (100×) (NEAA) (Thermo Fisher Scientific, Gibco, catalog number: 11140050)
HEPES (1 M) (Thermo Fisher Scientific, Gibco, catalog number: 15630130)
Sodium pyruvate (100 mM) (Thermo Fisher Scientific, Gibco, catalog number: 11360070)
Penicillin-streptomycin (10,000 U/mL) (Thermo Fisher Scientific, Gibco, catalog number: 15140122)
Blasticidin S hydrochloride, HEPES solution (Blasticidin) (10 mg/mL) (FUJIFILM Wako Pure Chemical Corporation, catalog number: 022-18713)
Opti-MEM I reduced serum medium (Thermo Fisher Scientific, Gibco, catalog number: 31985070)
Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific, Invitrogen, catalog number: L3000015)
Collagen-coated T225 flask (Corning, catalog number: NCO431082)
Dulbecco’s phosphate buffered saline (PBS) (Nacalai Tesque, catalog number: 14249-24)
Dimethyl sulfoxide (DMSO) (Nacalai Tesque, catalog number: 09659-14)
Syringe-top filter unit; Millex-HV Syringe Filter Unit, 0.45 μm, PVDF, 33 mm (Merck Millipore, catalog number: SLHVR33RS)
Amicon Ultra-15 centrifugal filter units (100 kDa) (Merck Millipore, catalog number: UFC910096)
Purification of HBVcc
Optiprep (60% iodixanol solution) (Serumwerk Bernburg, catalog number: AXS-1114542)
Open-top thinwall ultra-clear tube (14 mm × 89 mm) (Beckman Coulter, catalog number: 344059)
DNase; RQ1 RNase-free DNase (Promega, catalog number: M6101)
QIAamp DNA Mini kit (Qiagen, catalog number: 51306)
Lumipulse G HBsAg-Quant (Fujirebio, catalog number: 296851)
Lumipulse G HBcrAg (Fujirebio, catalog number: 294109)
Luna Universal qPCR Master Mix (New England Biolabs, catalog number: M3003)
Primers and probe for the real-time PCR targeting the HBs region:
Forward primer: 5′-CTTCATCCTGCTGCTATGCCT-3′
Reverse primer: 5′-AAAGCCCAGGATGATGGGAT-3′
Probe: 5′-FAM-ATGTTGCCCGTTTGTCCTCTAATTCCA-TAMRA-3′
TNE buffer (see Recipes)
Titration of HBVcc
Collagen-coated 96-well culture plate (Corning, catalog number: NCO3585)
Polyethylene glycol average mol wt 8,000 (PEG8000) (Sigma-Aldrich, catalog number: P2139)
4% paraformaldehyde phosphate buffer solution (4% PFA) (FUJIFILM Wako Pure Chemical Corporation, catalog number: 163-20145)
Block ACE (Bio-Rad, catalog number: BUF029)
Triton X-100 detergent (Calbiochem, Merck Millipore, catalog number: 648466)
Anti-HBc antibody; anti-hepatitis B virus core antigen IgG fraction (polyclonal) (AUSTRAL Biologicals, catalog number: HBP-023-9)
Goat anti-rabbit IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor Plus 555 (Thermo Fisher Scientific, catalog number: A327320
DAPI (4’,6-Diamidino-2-phenylindole dihydrochloride) (FUJIFILM Wako Pure Chemical Corporation, catalog number: 342-07431)
4% PEG8000 (see Recipes)
Recipes
HepG2-NTCPsec+ culture medium
DMEM
10% FBS
Penicillin-streptomycin (100 U/mL)
NEAA (1×)
HEPES (10 mM)
Sodium pyruvate (1 mM)
TNE buffer
150 mM NaCl
10 mM Tris HCl (pH 7.5)
1 mM EDTA
4% PEG8000
Dissolve in water to 4% (w/v) in a water bath at 60 °C.
Equipment
Refrigerated centrifuge with swing rotor (TOMY, model: AX-310)
Automated chemiluminescent enzyme immunoassay system (Fujirebio, model: LUMIPULSE G1200)
Ultracentrifuge apparatus, Optima L-90K with an SW-41 Ti rotor (Beckman Coulter, model: Optima L-90K)
Analytical balance (Sartorius Lab Instruments, model: SECURA124-1SJP)
Real-time PCR system (Thermo Fisher Scientific, model: StepOnePlus Real-Time PCR System)
Fluorescence microscope (KEYENCE, model: BZ-X710)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Murayama, A., Akari, H. and Kato, T. (2023). Production and Purification of Cell Culture–generated Hepatitis B Virus by Transient Transfection and Density Gradient. Bio-protocol 13(14): e4779. DOI: 10.21769/BioProtoc.4779.
分类
微生物学 > 微生物生物化学 > 蛋白质
细胞生物学 > 基于细胞的分析方法 > 病毒性感染
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










