- EN - English
- CN - 中文
In vitro Selection and in vivo Testing of Riboswitch-inspired Aptamers
受核开关启发的适体的体外筛选和体内测试
发布: 2023年07月05日第13卷第13期 DOI: 10.21769/BioProtoc.4775 浏览次数: 2323
评审: Gal HaimovichRakesh ChatrikhiAnonymous reviewer(s)
Abstract
Engineered aptamers for new compounds are typically produced by using in vitro selection methods. However, aptamers that are developed in vitro might not function as expected when introduced into complex cellular environments. One approach that addresses this concern is the design of initial RNA pools for selection that contain structural scaffolds from naturally occurring riboswitch aptamers. Here, we provide guidance on design and experimental principles for developing riboswitch-inspired aptamers for new ligands. The in vitro selection protocol (based on Capture-SELEX) is generalizable to diverse RNA scaffold types and amenable to multiplexing of ligand candidates. We discuss strategies to avoid propagation of selfish sequences that can easily dominate the selection. We also detail the identification of aptamer candidates using next-generation sequencing and bioinformatics, and subsequent biochemical validation of aptamer candidates. Finally, we describe functional testing of aptamer candidates in bacterial cell culture.
Key features
• Develop riboswitch-inspired aptamers for new ligands using in vitro selection.
• Ligand candidates can be multiplexed to conserve time and resources.
• Test aptamer candidates in bacterial cells by grafting the aptamer back onto its expression platform.
Graphical overview
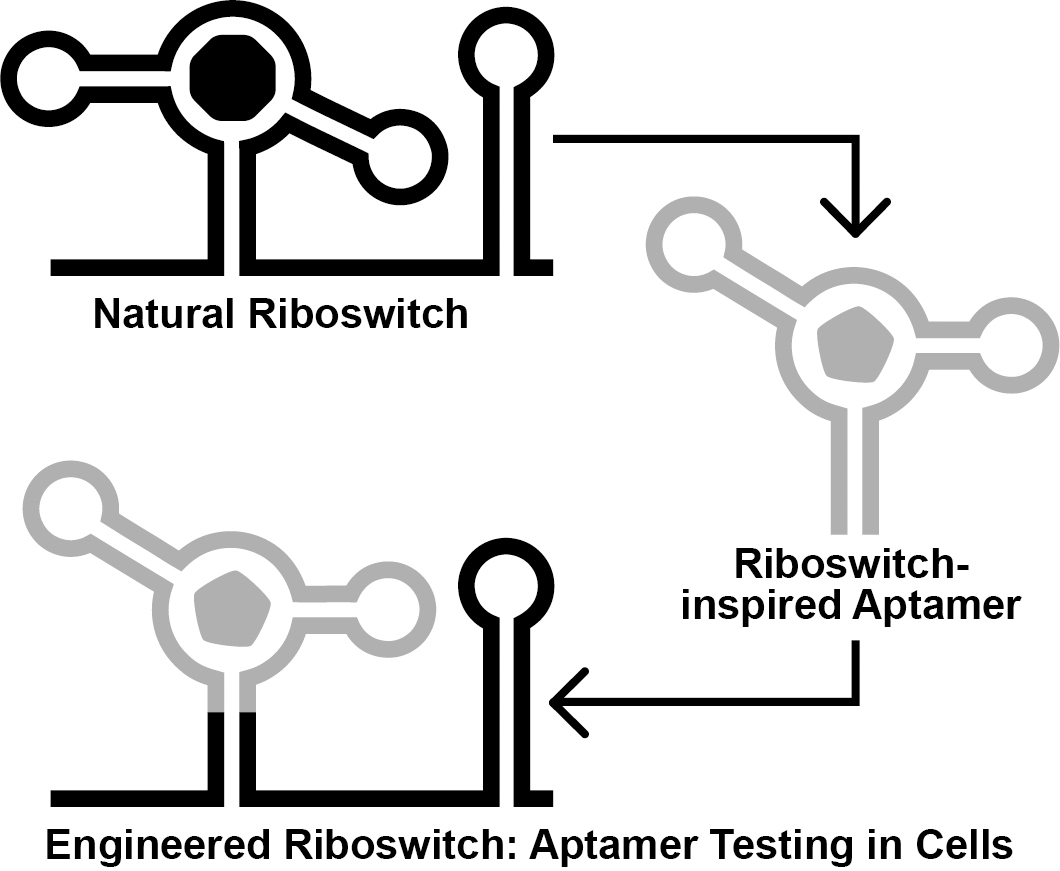
Background
Aptamers are ligand-binding oligonucleotides that are becoming increasingly useful for broad applications in diagnostics, therapeutics, and synthetic biology (Keefe et al., 2010; Topp and Gallivan, 2010; Wang et al., 2019). Aptamers occur naturally in the context of riboswitches, where they monitor the concentration of a target ligand and manipulate the folding of an adjoining expression platform to control the expression of their associated genes (Sherwood and Henkin, 2016; Kavita and Breaker, 2023). Engineered aptamers that bind different ligands can be developed using a technique called in vitro selection (Ellington and Szostak, 1990; Tuerk and Gold, 1990). This process involves generating a large combinatorial pool of oligonucleotides, selecting for those that bind a target ligand, and amplifying the selected oligonucleotides. This process is repeated iteratively until aptamers for the target ligand are identified.
Ideally, engineered aptamers could be useful for intracellular applications. However, the physiochemical conditions inside of a cell differ substantially from that of a test tube. Thus, aptamers developed in vitro might fail to perform inside of a cell, likely due to intrinsic factors such as the failure to reliably fold into the structure required to form the ligand binding pocket (Filonov et al., 2014). To address this, researchers have exploited the architectures of natural riboswitch aptamers to provide scaffolds for combinatorial RNA pools (Porter et al., 2017; Dey et al., 2022; Mohsen et al., 2023). We recently reported the Graftamer approach (Mohsen et al., 2023), in which engineered aptamers that contain a natural riboswitch scaffold are grafted back onto the natural expression platform (Figure 1). Using this approach, we developed aptamers for quinine and caffeine that retain the Guanine-I riboswitch (Mandal et al., 2003) scaffold from the initial combinatorial pool. These aptamers were each grafted back onto the expression platform of a Bacillus subtilis xpt-pbuX Guanine-I riboswitch. The resulting engineered quinine and caffeine riboswitches each display ligand-mediated gene regulation in B. subtilis cultures, indicating that the quinine and caffeine aptamers are functional in cells.
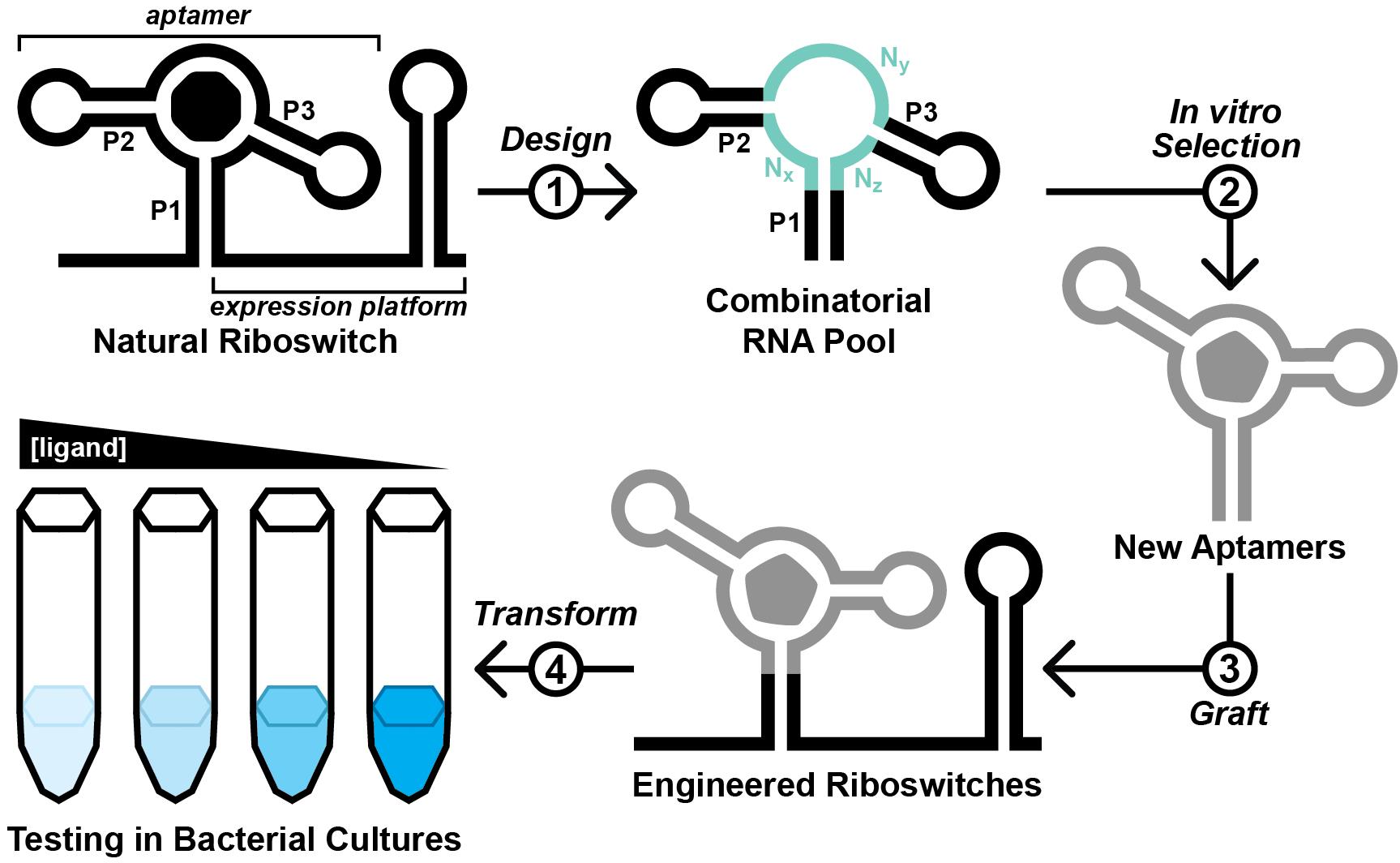
Figure 1. Overview of the Graftamer approach. First, a combinatorial RNA pool is designed by inserting regions of random RNA sequence (Nx, Ny, and Nz) in between structural features of a natural riboswitch aptamer. The depicted riboswitch contains an aptamer with paired elements P1, P2, and P3. Second, in vitro selection is performed to develop riboswitch-inspired aptamers that bind ligands different than that of the natural riboswitch. Third, validated aptamers that maintain the structural features of the natural riboswitch are grafted back onto the expression platform of the natural riboswitch to construct engineered riboswitches. Fourth, plasmids containing engineered riboswitches positioned upstream of a reporter gene are transformed into a suitable model organism. In the depicted example, the riboswitch functions as an OFF switch. Increased ligand concentration reduces the expression of a lacZ reporter gene, resulting in a corresponding decrease in blue color in the presence of X-gal indicator.
Here, we provide a protocol for this approach, beginning from the design and generation of the initial RNA pool. The selection process described herein is based on Capture-SELEX (Nutiu and Li, 2005; Stoltenburg et al., 2012; Yang et al., 2016; Lauridsen et al., 2018; Boussebayle et al., 2019), wherein the RNA pool is hybridized to a 3′-biotinylated DNA capture oligonucleotide, which itself is immobilized on a streptavidin-agarose column (Figure 2). In principle, RNA molecules that undergo a structural change upon binding a ligand are able to release from the capture oligonucleotide (Nutiu and Li, 2005). However, selfish molecules that slowly release from the capture oligonucleotide in a ligand-independent manner can jeopardize the selection (Mohsen et al., 2023). To counter the proliferation of selfish molecules, we recommend stringent washing and relatively short incubation times. Contamination between parallel lines of in vitro selection poses a threat as well. Thus, we suggest designing a different set of primers for each selection line performed in the same laboratory space. We anticipate that this protocol might have reduced utility for researchers pursuing aptamers of compounds that occur naturally in the target organism or that are unable to accumulate to appreciable intracellular concentrations (e.g., due to rapid efflux or metabolism, or an inability to permeate the cell wall or membrane). Nevertheless, we expect that this protocol will be broadly useful for improving best practices for in vitro selection and for increasing the likelihood of success for other researchers working to develop novel aptamers.
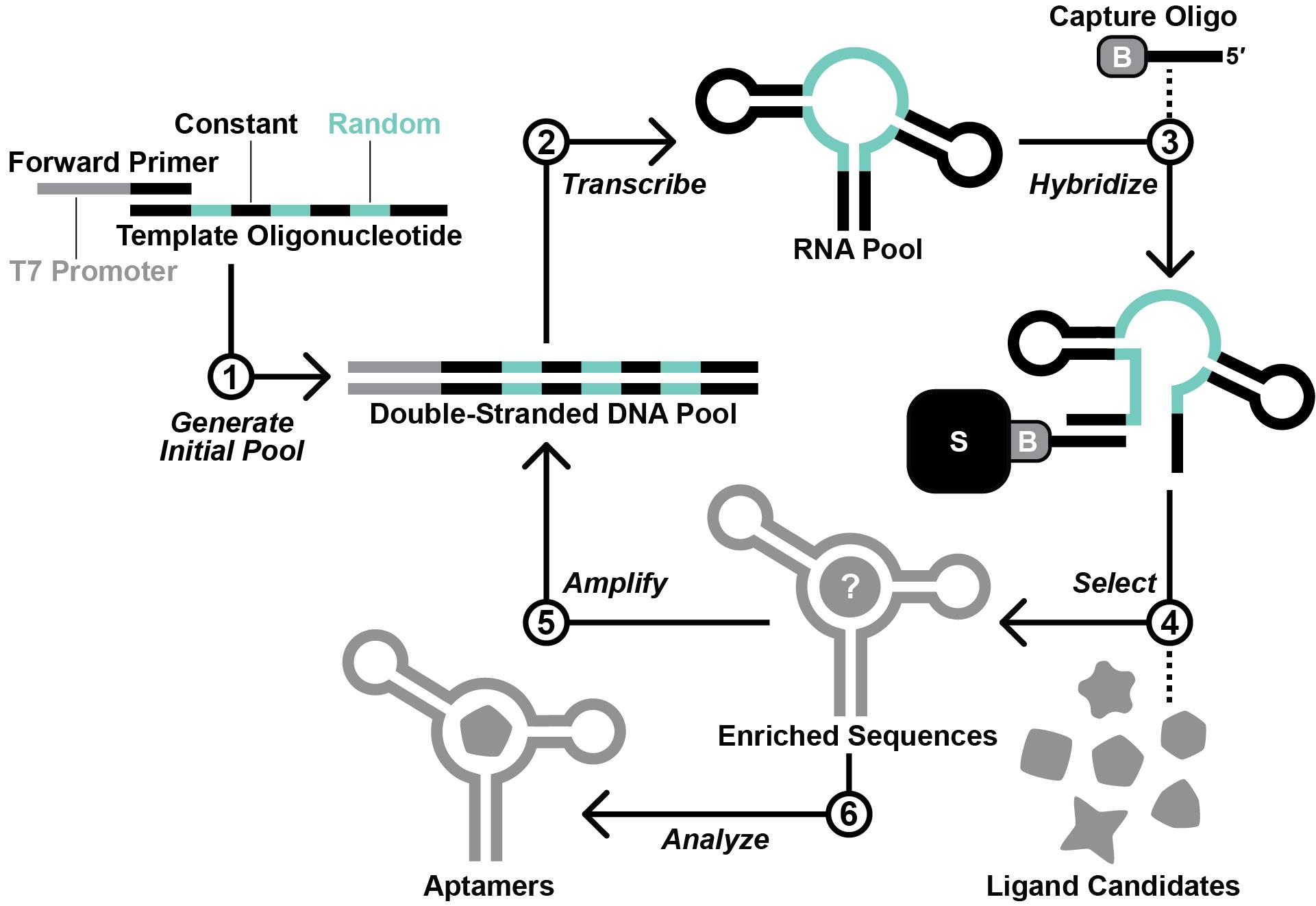
Figure 2. Overview of the in vitro selection scheme. First, the template oligodeoxynucleotide and forward primer are used to generate the initial DNA pool by primer extension. Second, in vitro transcription with T7 RNA polymerase is performed to produce the initial RNA pool. Third, the RNA pool is hybridized with a 3′-biotinylated DNA capture oligonucleotide. Fourth, RNA molecules that display affinity for a ligand candidate are isolated via a selection step. Fifth, the RNA molecules that survive the selection step are amplified by reverse transcription-polymerase chain reaction (RT-PCR). Steps 2–5 are repeated iteratively until the RNA pool is sufficiently enriched with functional aptamers. Finally, aptamer sequences are identified by sequencing and testing.
Materials and reagents
Biological materials
Bacillus subtilis strain 1A1 (American Type Culture Collection)
Escherichia coli strain BW25113 (Coli Genetic Stock Center at Yale University)
Reagents
Agarose (IBI Scientific, catalog number: IB70071)
Boric acid (Sigma, catalog number: B0394)
Bromophenol blue (Sigma, catalog number: B5525)
Dimethylformamide (Sigma, catalog number: 319937)
Dithiothreitol (DTT) (American Bioanalytical, catalog number: AB00490)
Ethanol, 200 proof HPLC grade (Sigma, catalog number: 459828)
Ethylenediaminetetraacetic acid (EDTA) (Sigma, catalog number: E5134)
Glacial acetic acid (J.T. Baker, catalog number: 9508-33)
HEPES [(4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] (American Bioanalytical, catalog number: AB00892)
Magnesium chloride hexahydrate (MgCl2·6H2O) (J.T. Baker, catalog number: 2444-01)
Potassium chloride (KCl) (Sigma, catalog number: 746436)
Purple 100 base pair DNA ladder (New England Biolabs, catalog number: N0551S)
Purple gel loading dye (6×) (New England Biolabs, catalog number: B7024S)
Sodium acetate (NaOAc) (Sigma, catalog number: 241245)
Sodium hydroxide (NaOH) pellets (J.T. Baker, catalog number: 3722-01)
Spermidine (Sigma, catalog number: S2501)
Sucrose (Sigma, catalog number: S0389)
SuperScript III Reverse Transcriptase (Invitrogen, catalog number: 18080093)
T7 RNA Polymerase (purified in-house; equivalent to New England Biolabs, catalog number: M0251L)
Taq DNA polymerase (New England Biolabs, catalog number: M0273L)
Trizma (Tris) base (Sigma, catalog number: T6066)
TURBO DNase (2 U/μL) (Invitrogen, catalog number: AM2238)
Urea (Sigma, catalog number: U5378)
UreaGel 19:1 concentrate (National Diagnostics, catalog number: EC-830)
UreaGel buffer (National Diagnostics, catalog number: EC-835)
UreaGel system diluent (National Diagnostics, catalog number: EC-840)
X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) (Cayman, catalog number: 16495)
Xylene cyanol FF (Sigma, catalog number: X-4126)
Solutions
2 M KCl (see Recipes)
5 M NaCl (see Recipes)
3 M NaOAc (see Recipes)
1 M MgCl2 (see Recipes)
1 M spermidine (see Recipes)
1 M Tris (pH 7.5 at ~20 °C) (see Recipes)
1 M dithiothreitol (DTT) (see Recipes)
X-gal (100 μg/mL) in dimethylformamide (see Recipes)
0.5 M EDTA (pH 8.0 at ~20 °C) (see Recipes)
Tris-borate-EDTA (TBE) (10×) (see Recipes)
Tris-acetate-EDTA (TAE) (50×) (see Recipes)
Transcription buffer (10×) (see Recipes)
Selection buffer (10×) (see Recipes)
Loading buffer (2×) (see Recipes)
Crush-soak buffer (see Recipes)
Recipes
2 M KCl
Add 14.9 g of KCl to a 100 mL flask. Add deionized H2O to 100 mL. Stir to mix. Filter-sterilize.
5 M NaCl
Add 29.2 g of NaCl to a 100 mL flask. Add deionized H2O to 100 mL. Stir to mix. Filter-sterilize.
3 M NaOAc
Add 24.6 g of NaOAc to a 100 mL flask. Add deionized H2O to 100 mL. Stir to mix. Filter-sterilize.
1 M MgCl2
Add 9.5 g of MgCl2 to a 100 mL flask. Add deionized H2O to 100 mL. Stir to mix. Filter-sterilize.
1 M spermidine
Add 145 mg of spermidine to a 1.5 mL microcentrifuge tube. Add 1 mL of dH2O. Vortex to mix.
Add 1 mL of deionized, sterile H2O (dH2O).
1 M Tris (pH 7.5 at ~20 °C)
Add 121.1 g of Tris to a 1 L flask. Add deionized H2O to ~900 mL. While stirring, adjust pH with hydrochloric acid to pH 7.5. Add deionized H2O to 1 L. Autoclave.
1 M dithiothreitol (DTT)
Add 154 mg of DTT to a 1.5 mL microcentrifuge tube. Add 1 mL of dH2O. Vortex to mix.
X-gal (100 μg/mL) in dimethylformamide
Add 100 mg of X-gal to a 1.5 mL microcentrifuge tube. Add 1 mL of dimethylformamide. Vortex to mix.
0.5 M EDTA (pH 8.0 at ~20 °C)
Add 186.1 g of EDTA to a 1 L flask. Add deionized H2O to ~800 mL. Stir to mix. Add 15 g of NaOH pellets. Stir to mix. While stirring, adjust pH with 10 N sodium hydroxide to pH 8.0. Add deionized H2O to 1 L. Autoclave.
TBE (10× concentrated, 4 L)
To a 4 L flask, add 432 g of Tris base, 220 g of boric acid, 14.9 g of EDTA, and deionized H2O to 4 L. Stir to mix. Filter particulates. Sterilize by autoclaving. The final solution is a 10× concentration buffer containing 0.9 M Tris, 0.9 M borate, and 10 mM EDTA pH 8.0 at ~20 °C.
TAE (50× concentrated, 1 L)
To a 1 L flask, add 242 g of Tris base, deionized H2O to ~800 mL, 57.1 mL of glacial acetic acid, and 100 mL of 0.5 M EDTA pH 8.0 at ~20 °C. Add deionized H2O to 1 L. Filter particulates. Sterilize by autoclaving. The final solution is a 50× concentrated buffer containing 2 M Tris, 1 M acetate, and 50 mM EDTA pH 8.0 at ~20 °C.
Transcription buffer (10× concentrated, 1 mL)
Mix 150 μL of 1 M MgCl2, 20 μL of 1 M spermidine, 500 μL of 1 M Tris (pH 7.5 at ~20 °C), 50 μL of 1 M DTT, and 280 μL of dH2O in a 1.5 mL microcentrifuge tube. 1× mixture: 150 mM MgCl2, 20 mM spermidine, 500 mM Tris (pH 7.5 at ~20 °C), and 50 mM DTT. Store at -20 °C
Selection buffer (10× concentrated, 100 mL)
Mix 4.77 g of HEPES, 50 mL of 2 M KCl, 1 mL of 1 M MgCl2, and 35 mL of deionized H2O together in a flask containing a magnetic stir bar. Adjust the pH of the solution to 7.4 by adding pre-weighed NaOH pellets while stirring. Once a pH of 7.4 is attained, remove any remaining NaOH pellets with a clean spatula. Dry and weigh the pellets. After calculating the amount of NaOH required to adjust the pH (typically ~70 mM), add a volume (typically ~0.6 mL) of 5 M NaCl to the solution to bring the total [Na+] to 100 mM. Add deionized H2O to adjust the final volume of the solution to 100 mL. Filter-sterilize the resulting solution. The final solution is a 10× concentrated selection buffer containing 200 mM HEPES, 1 M KCl, 30 mM NaCl (total [Na+]: 100 mM), and 10 mM MgCl2, pH 7.4 at ~20 °C [1×: 20 mM HEPES, 100 mM KCl, 3 mM NaCl (total [Na+]: 10 mM), and 1 mM MgCl2]. HEPES is light-sensitive, so store this buffer in a darkened compartment (cabinet, refrigerator, etc.).
Loading buffer (2× concentrated, 40 mL)
Add 44 g of urea to a flask and add 28 mL of dH2O. Stir with gentle heat until urea is dissolved. Add 8 g of sucrose, 20 mg of bromophenol blue, 20 mg of xylene cyanole FF, 0.4 mL of 10% SDS, and 4 mL of 10× TBE. Stir with gentle heat until sucrose is dissolved. Store at 4 °C.
Crush-soak buffer
To a 1 L flask, add 40 mL of 5 M NaCl, 10 mL of 1 M Tris (pH 7.5 at ~20 °C), and 2 mL of 0.5 M EDTA (pH 8.0 at ~20 °C). Add deionized H2O to 1 L. Autoclave.
Laboratory supplies
0.5 mL low adhesion microcentrifuge tubes (USA Scientific, catalog number: 1405-2600)
1.5 mL low adhesion microcentrifuge tubes (USA Scientific, catalog number: 1415-2600)
8-strip 0.2 mL PCR tubes (Dot Scientific, catalog number: 401)
Gel-loading pipette tips (VWR, catalog number: 76321-828)
Individual PCR tubes 8-tube strip, clear (Bio-Rad, catalog number: TLS0801)
iTaq Universal SYBR Green Supermix (Bio-Rad, catalog number: 1725121)
Luer-Lok 3 mL syringe (BD, catalog number: 309657)
Micro Bio-SpinTM chromatography columns (Bio-Rad, catalog number: 7326204)
Optical flat 8-cap strips for 0.2 mL tube strips (Bio-Rad, catalog number: TC20803)
PierceTM Streptavidin agarose (Thermo Scientific, catalog number: 20353)
PrecisionGlide needle 27 G × 1/2 (BD, catalog number: 305109)
QIAquick PCR Purification kit (Qiagen, catalog number: 28104)
Sterile pipette tips with filters (filters help to avoid contamination)
Vivaspin 500 molecular weight cutoff 10 kDa (Cytiva, catalog number: 28932225)
Equipment
Analytical balance (Mettler Toledo, catalog number: AG285)
Cary 60 UV-Vis spectrophotometer (originally Varian, now Agilent)
CFX Opus 96 Real-Time PCR System (Bio-Rad, catalog number: 12011319)
Geiger counter (Ludlum)
Gel Doc Go Gel imaging system (Bio-Rad, catalog number: 12009077)
Handheld shortwave (254 nm) ultraviolet lamp (UVP, catalog number: UVG-65)
Innova 42R shaking incubator (Eppendorf, catalog number: M1335-0004)
Mastercycler Nexus GX2 (Eppendorf, catalog number: 6336000015)
Milli-Q Advantage A10 (Millipore-Sigma, catalog number: Z00Q0V0WW)
Mini centrifuge (Thermo Fisher, catalog number: 75004061)
NanoDrop 8000 spectrophotometer (Thermo Fisher, catalog number: ND-8000)
Orion Star A211 Benchtop pH meter (Thermo Fisher, catalog number: STARA2110)
Phosphorimager Typhoon FLA 9500 (originally GE Healthcare, now Cytiva)
PowerPac HV power supply (Bio-Rad, catalog number: 1645056)
Slab Gel Dryer Model 583 (Bio-Rad, catalog number: 165-1745)
Sorvall Legend Micro 21R refrigerated centrifuge (Fisher Scientific, catalog number: 75002446)
Speed Vac (originally Thermo-Savant, now Thermo Fisher)
Standard set of pipettes that can transfer volumes in the range of 1–1,000 μL
Synergy Neo2 Multimode Reader (originally BioTek, catalog number: 1351000, now Agilent)
Vortex-Genie 2 (Scientific Industries, catalog number: SI-0236)
Software
R2R version 1.0.6, 2018-12-10 (https://sourceforge.net/projects/weinberg-r2r/)
CMfinder version 0.4.1.18, 2019-04-22 (https://sourceforge.net/projects/weinberg-cmfinder/)
Scripts toTally.py and selfishCluster.py, 2023-01-09 (Mohsen et al., 2023)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Mohsen, M. G. and Breaker, R. R. (2023). In vitro Selection and in vivo Testing of Riboswitch-inspired Aptamers. Bio-protocol 13(13): e4775. DOI: 10.21769/BioProtoc.4775.
分类
生物工程 > 合成生物学 > 核酸适配体
分子生物学 > RNA > RNA 结构
分子生物学 > RNA
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













