- EN - English
- CN - 中文
Visualizing NBD-lipid Uptake in Mammalian Cells by Confocal Microscopy
共聚焦显微镜观察哺乳动物细胞对NBD脂质的摄取
发布: 2023年07月05日第13卷第13期 DOI: 10.21769/BioProtoc.4771 浏览次数: 2414
评审: Jan HuebingerShalini Low-NamMario Ruiz
Abstract
Eukaryotic cells use a series of membrane transporters to control the movement of lipids across their plasma membrane. Several tools and techniques have been developed to analyze the activity of these transporters in the plasma membrane of mammalian cells. Among them, assays based on fluorescence microscopy in combination with fluorescent lipid probes are particularly suitable, allowing visualization of lipid internalization in living cells. Here, we provide a step-by-step protocol for mammalian cell culture, lipid probe preparation, cell labeling, and confocal imaging to monitor lipid internalization by lipid flippases at the plasma membrane based on lipid probes carrying a fluorophore at a short-chain fatty acid. The protocol allows studying a wide range of mammalian cell lines, to test the impact of gene knockouts on lipid internalization at the plasma membrane and changes in lipid uptake during cell differentiation.
Key features
•Visualization and quantification of lipid internalization by lipid flippases at the plasma membrane based on confocal microscopy.
•Assay is performed on living adherent mammalian cells in culture.
•The protocol can be easily modified to a wide variety of mammalian cell lines.
Graphical overview
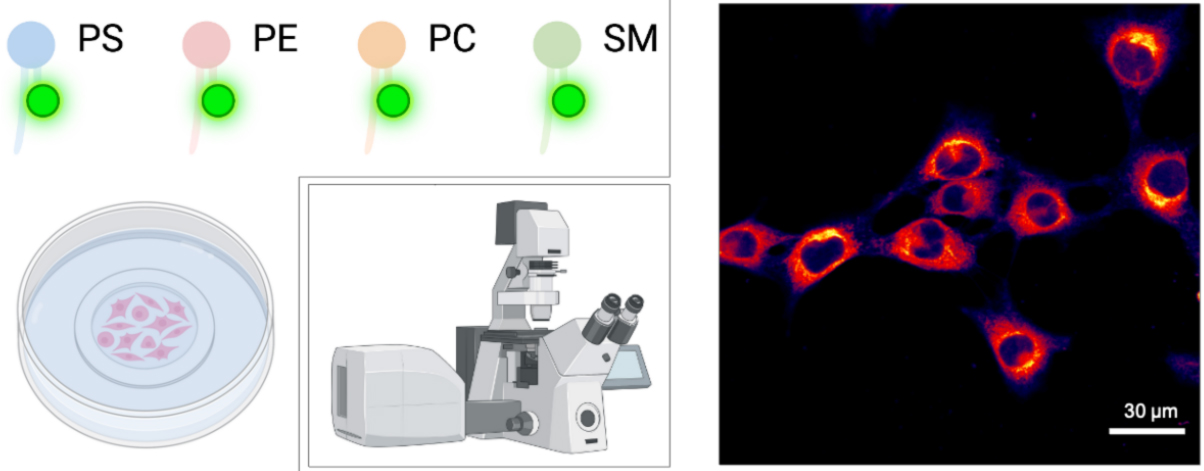
Analysis of NBD-lipid uptake in adherent mammalian cells by confocal microscopy. Scale bar, 30 μm.
Background
Eukaryotic cells use a series of membrane transporters to control the movement of lipids across their plasma membrane. These transporters can be divided into ATP-dependent flippases and floppases—which catalyze the inward movement of phospholipids from the extracellular/luminal leaflet to the cytoplasmic leaflet and the outward movement of lipids, respectively—and ATP-independent scramblases (Holthuis and Levine, 2005; Contreras et al., 2010). Several tools and techniques have been developed to analyze the activity level of these transporters in the plasma membrane of mammalian cells. Among them, assays based on fluorescent lipid probes are particularly suitable, allowing visualization and quantification of lipid internalization by flippases in living cells. Lipid probes bearing a fluorophore such as nitrobenzoxadiazole (NBD) on a short-chain fatty acid at the sn-2 position, together with a long fatty acid residue at the sn-1 position, are most commonly used (Martin and Pagano, 1987; Koval and Pagano, 1991; Hoekstra and Kok, 1992; Rosenwald and Pagano, 1993) (Figure 1). Both BODIPY- and pyrene-labeled lipids have been used to track lipid trafficking in cells (Pagano et al., 1999; Somerharju, 2002), each having advantages and disadvantages (Table 1). Since the lipid polar head group stays unmodified, recognition as a substrate by ATP-dependent flippases and floppases is not affected (Theorin et al., 2019). At the same time, these molecules are less hydrophobic than their naturally occurring counterparts and readily insert into cell membranes when added to the medium. Transport of these short-chain lipid probes is usually monitored by extraction with bovine serum albumin (BSA) of the residual fraction of analogs not transported across the plasma membrane. As BSA extracts all analogs from the exoplasmic monolayer of the plasma membrane, the inaccessible fraction reflects analogs that have been internalized into cells. Alternatively, NBD-labeled lipids on the outer monolayer can be selectively destroyed with the water-soluble quencher dithionite (McIntyre and Sleight, 1991). However, because dithionite can leak through the membrane in some cells, conditions must be carefully adapted to the particular cell type (Pomorski et al., 1994). Furthermore, NBD-lipids are known to be actively metabolized by phospholipase activities. One frequent modification is their hydrolysis into lyso-derivates by phospholipase A2 activities, which results in the removal of the fatty acid attached to the sn-2 position. The liberated labeled C6 fatty acids are released into the medium, hampering the quantitative analysis of NBD-lipid internalization. Thus, the assay is typically performed in the presence of phospholipase inhibitors (Pomorski et al., 1996; Grifell-Junyent et al., 2022; Herrera et al., 2022).
The protocol presented here utilizes fluorescence microscopy to study NBD-lipid internalization in mammalian cells, exemplified on mouse skeletal muscle cell line (C2C12) cells (Grifell-Junyent et al., 2022), and has also been applied by us to fibroblasts (Pomorski et al., 1996). While NBD-lipids offer advantages (Table 1), including the ability to monitor the activity of lipid flippases at the plasma membrane of cells, it is important to note that these probes are less hydrophobic than naturally occurring lipids. This difference in hydrophobicity can affect the way they are transported within cells and may lead to differences in their intracellular trafficking compared to endogenous lipids. The protocol includes cell preparation, preparation of NBD-lipids, labeling of cells with NBD-lipids, fluorescence microscopy, and data analysis. The protocol can be easily adapted to parasites such as Toxoplasma and Leishmania (Weingärtner et al., 2011; Chen et al., 2021). It can also be applied to study: (i) the lipid uptake profile in mammalian cell lines; (ii) the impact of gene deletions and/or single mutations in lipid uptake at the plasma membrane; and (iii) the possible changes in lipid uptake during cell differentiation. For lipid uptake assays optimized for plants or based on flow cytometry, the reader is referred to previously published protocols (Jensen et al., 2016; López-Marqués and Günther Pomorski, 2021; Herrera et al., 2022).
Table 1. Main advantages and disadvantages of common fluorescently tail-labeled lipid probes
| Lipid probe | Advantages | Disadvantages | Reference |
|---|---|---|---|
| NBD-lipids | • Commercially available • Dithionite quenchable | • Low photostability • High environmental sensitivity | Martin and Pagano, 1987; Kobayashi and Arakawa, 1991; Pomorski et al., 1996) |
| BODIPY and TopFluor lipids | • High photostability • Low environmental sensitivity • Superior brightness • MαCD-mediated lipid exchange | • Availability restricted • Poorly extractable by BSA | (Pagano et al., 1999; Kay et al., 2012; Mioka et al., 2018; Segawa et al., 2021) |
Pyrene lipids | • Mimic natural lipids • Unique spectral features | • UV excitation • Low photostability • Availability restricted • Poorly extractable by BSA • High environmental sensitivity | Tanhuanpää et al., 2000; Somerharju, 2002 |
Abbreviation: MαCD, methyl-α-cyclodextrin
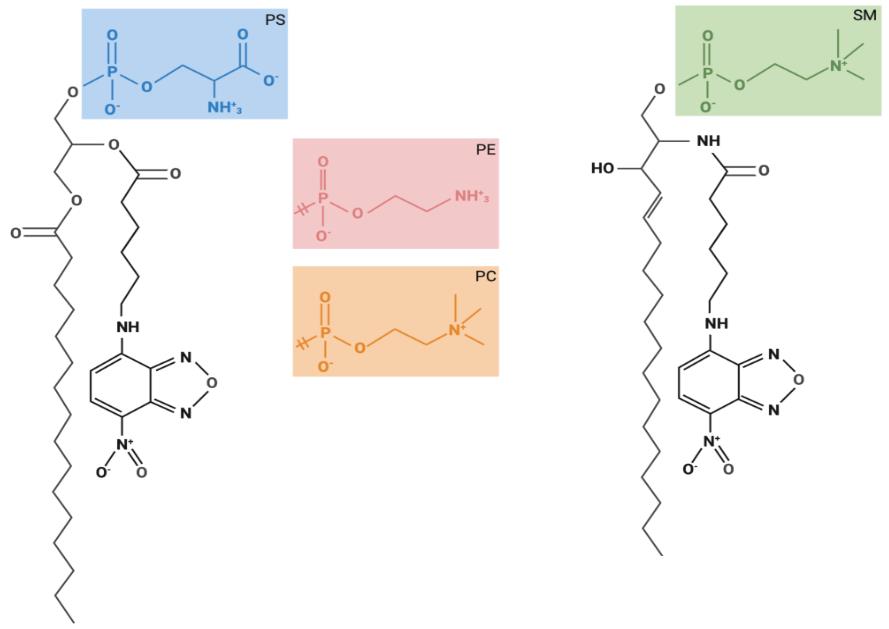
Figure 1. Chemical structures of fluorescent lipids used for lipid uptake assays. Glycerophospholipids are composed of two hydrophobic fatty acids and a hydrophilic head group, both combined with a glycerol backbone. Sphingolipids are composed of one hydrophobic fatty acid and a hydrophilic head group, both combined via a sphingosine backbone. The structures of the glycerophospholipid head groups corresponding to phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylserine (PS), as well as for sphingomyelin (SM) are depicted. These fluorescently labeled lipids carry a nitrobenzoxadiazole (NBD) group at the sixth carbon in the short-chain fatty acid at the sn-2 position.
Materials and reagents
Mammalian cell culture
In this study, we used mouse myoblast cells (C2C12; cell number: ACC 565, DSMZ Braunschweig, Germany) that were cultured in growth medium (see Recipes). Optimal culture media and conditions may differ for other cell lines.
Dulbecco's Modified Eagle Medium, high glucose, without pyruvate (high-glucose DMEM) (e.g., Sigma-Aldrich, catalog number: D5796), store at 4 °C
Dulbecco's Modified Eagle Medium, low glucose, without pyruvate (low-glucose DMEM) (e.g., Sigma-Aldrich, catalog number: D6046), store at 4 °C
Ethanol absolute ≥ 99.8% (VWR, catalog number: 20821.321)
Fetal bovine serum, heat inactivated before use (FBS) (e.g., Capricorn Scientific, catalog number: FBS-11A), store at -20 °C
Hanks’ balanced salt solution, Ca2+ and Mg2+ free (HBSS) (e.g., Sigma-Aldrich, catalog number: H6648), store at 4 °C
Horse serum (HS) (e.g., Sigma-Aldrich, catalog number: H1138), store at -20 °C
35 mm polymer bottom dishes (e.g., Ibidi, catalog number: 81156)
1.5 mL microcentrifuge tubes (Sarstedt, catalog number: 72.690.001)
Penicillin-streptomycin, 100× solution (e.g., Sigma-Aldrich, catalog number: P4333), store at -20 °C
Pipette controller (e.g., accu-jet pro, Brand, catalog number: 263 00)
Polypropylene tubes of 15 mL capacity (e.g., Falcon tubes, Sarstedt, catalog numbers: 62.554.502 and 62.547.254)
Sterile serological pipettes (e.g., Serological pipettes of 5, 10, and 25 mL; Sarstedt, catalog numbers: 86.1253.001, 86.1254.001, and 86.1685.001)
Sterile culture vessels T-75 flasks (e.g., Sarstedt, catalog number: 83.3911)
Trypsin-EDTA solution (e.g., Sigma-Aldrich, catalog number: T3924), store at -20 °C
Trypan blue solution, 0.4% (Thermo Fischer Scientific, catalog number: 15250061)
Tyrode’s balanced salt solution (TBSS) (see Recipes), store at 4 °C
Preparation of NBD-lipids
Centrifuge glasses DURAN® with conical bottom, 12 mL (Carl Roth, catalog number: K211.1)
Chloroform 99%–99.4% ethanol-stabilized and certified for absence of phosgene and HCl (VWR, catalog number: 22711.290)
Methanol ≥ 99.8% (VWR, catalog number: 20847)
Glass vials (Rotilabo® screw neck ND8 vials, Brown glass, 1.5 mL, Carl Roth, Karlsruhe, Germany, catalog number: KE30.1) with screw caps (without borehole, without septum, PP, black, ND8, Carl Roth, Karlsruhe, Germany, catalog number: KE39.1) for lipid aliquoting
C16:0-C6:0 NBD-lipids purchased in chloroform including NBD-PC (Avanti Polar Lipids, catalog number: 810130), NBD-PE (Avanti Polar Lipids, catalog number: 810153), NBD-PS (Avanti Polar Lipids, catalog number: 810192), and NBD-SM (Avanti Polar Lipids, catalog number: 810218)
NBD-lipid uptake assay
Bovine serum albumin essentially fatty acid free (BSA) (Sigma-Aldrich, catalog number: A6003), store at 4 °C
Calcium chloride (CaCl2) (Grüssing, catalog number: 10043-52-4)
Dimethyl sulfoxide (DMSO) (Carl Roth, catalog number: 4720.4)
Glucose (Duchefa Biochemie, catalog number: G0802.5000)
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES) (Carl Roth, catalog number: 7365-45-9)
Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma-Aldrich, catalog number: 7791-18-6)
3-(4-octadecyl)benzoylacrylic acid, 4-(4-octadecylphenyl)-4-oxo-2-Butenoic acid (OBAA) (Sigma-Aldrich, catalog number: SML0075), store at -80 °C
Phenylmethylsulfonyl fluoride (PMSF) (Sigma-Aldrich, catalog number: P7626)
Note: OBAA is a potent inhibitor of phospholipase A2 (Eintracht et al., 1998). PMSF has been reported to inhibit phospholipases as well as some esterases (James, 1978; Gadella and Harrison, 2000; Estévez et al., 2012).
Potassium chloride (KCl) (Merck, catalog number: 7447-40-7)
Sodium chloride (NaCl) (Carl Roth, catalog number: 7647-14-5)
Sodium dihydrogen phosphate (NaH2PO4·2H2O) (VWR chemicals, catalog number: 28015.294)
Sodium hydroxide (NaOH) (Merck, catalog number: 30620-1KG-M)
Media and buffers
Growth medium (see Recipes)
Differentiation medium (see Recipes)
TBSS buffer (see Recipes)
NBD-lipid stocks (see Recipes)
PMSF stock of 200 mM (see Recipes)
OBAA stock of 5 mM (see Recipes)
BSA solution in TBSS (see Recipes)
Equipment
Autoclave sterilizer (e.g., Systec VX-65, Systec, Linden, Germany)
Biological safety cabinet certified for handling of biological materials (e.g., Herasafe KSP Class II Biological Safety Cabinets, Thermo Fisher Scientific)
Centrifuge with rotor for 15 mL polypropylene tubes (e.g., Eppendorf 5810 R; Wesseling, Germany)
Computer with monitor (e.g., DELL U2415)
Confocal laser scanning microscope (e.g., Leica TCS SP8)
Eppendorf Research® plus pipettes P2, P20, P200, P1000 (Eppendorf, catalog numbers: 3123000012, 3123000039, 3123000055, 3123000063)
Flow cabinet to work with organic solvents
Freezers -20 °C and -80 °C
Glass desiccator Boro 3.3 with socket in lid, 20 cm, including stopcock (BRAND, catalog number: 65238)
Hamilton 700 Series Syringes 10, 25, 100, and 1,000 μL (Hamilton Company, Nevada, USA)
Incubator with humidity and gas control to maintain 37 °C and 95% humidity in an atmosphere of 5% CO2 in air (e.g., Binder, Tuttlingen, Germany)
Incubator to maintain 20 °C (e.g., incubator with Peltier elements heating up and cooling down seamlessly in one system, Memmert, Schwabach, Germany)
Inverted phase contrast microscope equipped with a 10× objective (HI PLAN I 10×/0.22 PH1; Leica DMi1, Mannheim, Germany)
Neubauer counting chamber (improved dark lines, 0.1 mm) and cover glasses (20 × 26 × 0.4 mm)
Pipette tips 2, 10, 200, and 1,000 μL (Sarstedt, catalog numbers: 70.1130.212, 70.760.002, 70.3030.020, and 70.3050.020)
Refrigerator
Tubing (BRAND, catalog number: 143275)
Vortex mixer (e.g., Vortex Genie 2 Scientific Industries Inc., catalog number: SI-0236)
Vacuum Pump V-100 with Interface I-100 (Buchi, Switzerland, catalog numbers: 11593636 and 11593655D)
Water distillation system
Water bath (e.g., WPE45 Memmert, Schwabach, Germany) for mammalian cells and for NBD-lipid labeling (e.g., Julabo CORIO C-BT5, catalog number: 9011305)
Software
ImageJ (Wayne, Rasband, S., U. S. National Institutes of Health, Bethesda, Maryland, USA, https://imagej.nih.gov/ij/index.html, version v.153q)
Leica Application Suite AF (LAS X 3.5.7.23225, Leitz, Wetzlar, Germany)
Leica’s LAS X Office (LASX Office, ver. 1.4.4 26810, Leica, Wetzlar, Germany)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Baum, J. F., Bredegaard, L., Herrera, S. A. and Pomorski, T. G. (2023). Visualizing NBD-lipid Uptake in Mammalian Cells by Confocal Microscopy. Bio-protocol 13(13): e4771. DOI: 10.21769/BioProtoc.4771.
分类
细胞生物学 > 细胞结构 > 细胞质膜
生物化学 > 脂质 > 脂质转运
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link