- EN - English
- CN - 中文
Improved Methods for Acetocarmine and Haematoxylin Staining to Visualize Chromosomes in the Filamentous Green Alga Zygnema (Charophyta)
用醋酸洋红和苏木精染色法观察丝状绿藻Zynema(轮藻门)染色体的改进方法
发布: 2023年08月20日第13卷第16期 DOI: 10.21769/BioProtoc.4768 浏览次数: 1446
评审: Dennis J NürnbergMercedes Nieves MoriónShuhei Ota
Abstract
Genome sizes of Zygnema spp. vary greatly, being unknown whether polyploidization occurred. The exact number of chromosomes in this genus is unknown since counting methods established for higher plants cannot be applied to green algae. The massive presence of pectins and arabinogalactan proteins in the cell wall interferes with the uptake of staining solutions; moreover, cell divisions in green algae are not restricted to meristems as in higher plants, which is another limiting factor. Cell divisions occur randomly in the thallus, due to the intercalary growth of algal filaments. Therefore, we increased the number of cell divisions via synchronization by changing the light cycle (10:14 h light/dark). The number of observed mitotic stages peaked at the beginning of the dark cycle. This protocol describes two methods for the visualization of chromosomes in the filamentous green alga Zygnema. Existing protocols were modified, leading to improved acetocarmine and haematoxylin staining methods as investigated by light microscopy. A freeze-shattering approach with liquid nitrogen was applied to increase the accessibility of the haematoxylin dye. These modified protocols allowed reliable chromosome counting in the genus Zygnema.
Key features
• Improved method for chromosome staining in filamentous green algae.
• Optimized for the Zygnema strains SAG 698-1a (Z. cylindricum), SAG 698-1b (Z. circumcarinatum), and SAG 2419 (Zygnema ‘Saalach’).
•This protocol builds upon the methods of chromosomal staining in green algae developed by Wittmann (1965), Staker (1971), and Fujii and Guerra (1998).
• Cultivation and synchronization: 14 days; fixation and permeabilization: 24 h; staining: 1 h; image analysis and chromosome number quantification: up to 20 h.
Keywords: Acetocarmine (醋酸洋红)Background
Background on chromosome counting in Zygnema
Zygnematophyceae are the immediate sister group to land plants, thought to have colonized land 550 million years ago (Wodniok et al., 2011; Leebens-Mack et al., 2019). Data on nuclear DNA content are available for Zygnema (Čertnerová, 2021; Feng et al., 2021 and 2023), and genome sizes for different strains of Zygnema spp. have been established, but these show great variation (Feng et al., 2023). Chromosomes of Zygnema have previously been illustrated by transmission electron microscopy (Bakker and Lokhorst, 1987), although without giving actual numbers. The actual chromosome numbers of Zygnema vary drastically, ranging from 14 to 82 (Kurssanow, 1911; Miller 1973; Guiry et al., 2022). Thus, it is unclear if polyploidization has happened. Traditional counting methods established for higher plants are hampered in green algae by pectins [massive homogalacturonan accumulations have been reported by Herburger et al. (2019)] and arabinogalactan proteins in the cell wall. Even though numerous protocols have been established for chromosomal staining in green algae (Godward, 1948; Wittmann, 1965; Prasad and Godward, 1966; Staker, 1971; Gerlach, 1977; Fujii and Guerra, 1998), they still face difficulties handling Zygnema spp., as their chromosomes are small, sticky, and mostly only able to be counted in their mid- to late-prophase. Furthermore, conventional staining methods lead to over-staining of the cell wall and DNA-dense areas in the cytoplasm known as karyoide in Zygnema, Charophyta (Kopetzky-Rechtperg, 1934).
Modification of the methods from Wittmann (1965), Staker (1971), and Fujii and Guerra (1998)
For all three Zygnema ssp., two different modified staining procedures were used: (1) acetocarmine (Figure 1A–1E) and (2) haematoxylin (Figure 1A, 1F–1L). To achieve full mitotic synchronization in Zygnema, a light/dark cycle (10:14 h) was applied following Staker (1971), i.e., with a longer dark period than our standard cultivation cycle (light/dark, 16:8 h).
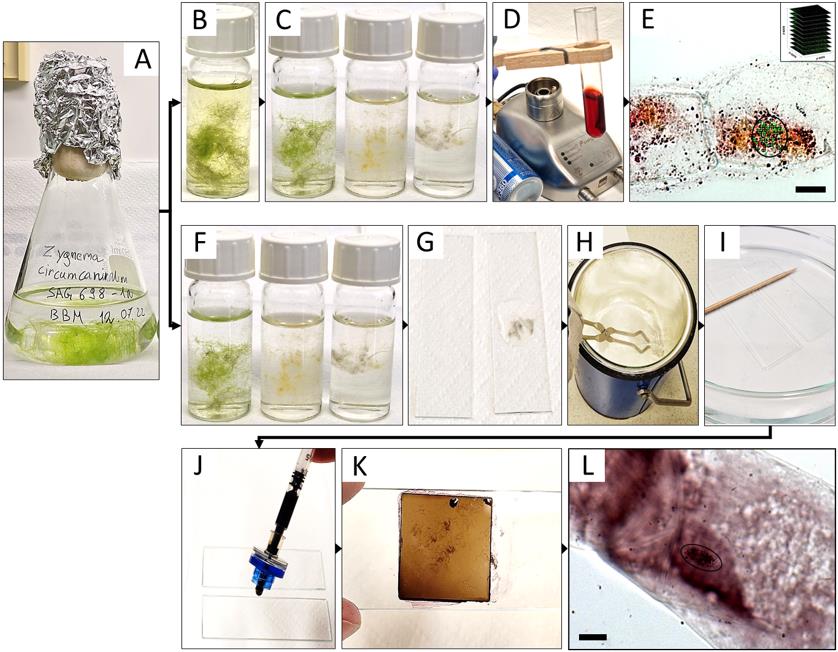
Figure 1. Summary of the experimental procedure for acetocarmine (B–E) and haematoxylin (F–L) staining in Zygnema circumcarinatum.(A) Erlenmeyer flask with young culture; (B) 8-hydroxyquinoline prefixation; (C, F) fixation in Carnoy’s fluid, illustrating the gradual bleaching from time 0, 1, and 12 h (from left to right); (D) staining with acetocarmine; (E) projected Z-stack rendered by Helicon Focus software used for counting the chromosomes (circle: chromosomes marked with green crosses in ImageJ; inset: symbolic representation of individual images for Z-stacks); (G) algal biomass mounted in acetic acid and squished between two slides; (H) slides dipped in liquid nitrogen; (I) HCl treatment; (J, K) aceto-haematoxylin-iron alum staining; (L) light microscopic image used for quantifying the number of chromosomes (circle). Scale bars = 10 µm.
For the staining of the chromosomes, Staker (1971) used propriocarmine, which was replaced with 1% acetocarmine in the current protocol; also, the Zygnema filaments were not chopped or randomized, which would lead to complete destruction. Moreover, prior to the fixation in Carnoy’s fluid, cells were treated with 8-hydroxyquinoline to depolymerize microtubules, resulting in sticky and condensed metaphase chromosomes (Bukhari, 2004). Haematoxylin staining by Wittmann (1965) was used, but the treatment with chloralhydrate and slide heating steps was omitted and replaced with hydrolysis by 5 N HCl (Fujii and Guerra, 1998), resulting in contrast improvement between chromosomes and cytoplasm. Changes only occurred with the algal filaments being placed between two microscopic slides before liquid nitrogen treatment with a freeze-shattering method (Wasteneys et al., 1997); the HCl treatment time was reduced to 10 min to assure the integrity of the Zygnema filaments, which disintegrate after prolonged treatment.
Materials and reagents
Biological material
Zygnema cylindricum strain SAG 698-1a (Figure 2A; Feng et al., 2021; isolated 1929 by Czurda V.; deposited 1954 by Pringshein E.G.), collected from a ditch at meadow Poselteich (Polenský Rybnik; 50°33′09.7″N 14°40′09.7″E) near Hirschberg (Dosky) in Czech Republic, Europe.
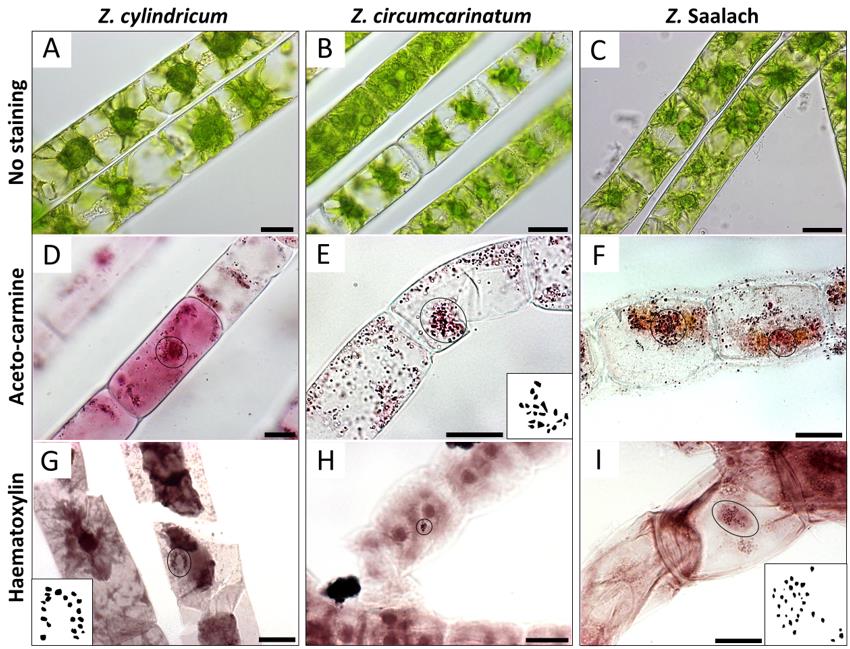
Figure 2. Chromosomes (circles) visualized by light microscopy in different Zygnema strains. (A, D, G) Z. cylindricum; (B, E, H) Z. circumcarinatum; and (C, F, I) Z. Saalach. (A, B, C) Living cells without staining; (D, E, F) acetocarmine staining; (G, H, I) haematoxylin staining. Insets in E, G, and I show manual drawings of chromosomes counted with ImageJ; counted chromosome numbers: n = 20 for Z. cylindricum/Z. circumcarinatum and n = 30 for Z. Saalach. Scale bars = 20 µm.Zygnema circumcarinatum strain SAG 698-1b (Figure 2B; isolated 1929 by Czurda V.; deposited 1954 by Pringshein E.G.), collected from a ditch at meadow Poselteich (Polenský Rybnik; 50°33′09.7"N 14°40′09.7″E) near Hirschberg (Dosky) in Czech Republic, Europe. The results of the chromosome counting for this strain have been recently published (Feng et al., 2023)
Zygnema ‘Saalach’ (SAG 2419; 47°47′8.70″N, 12°56′42.66″E; 440 m above sea level; Figure 2C), collected near Salzburg, Austria (Herburger et al., 2015)
Reagents
8-Hydroxyquinoline (Merck, catalog number: 148-24-3)
Acetocarmine (Morphisto, catalog number: 10411)
Liquid nitrogen
5 N HCl (Merck, catalog number: 258148)
Bi-distilled water (A. bidest)
Bold’s basal culture medium (BBM), pH 5.5 (Bischoff and Bold, 1963)
Glacial acetic acid (Merck, catalog number: A6283)
100% ethanol (Sigma, catalog number: 493546)
45% acetic acid (Merck, catalog number: A6283)
Haematoxylin (Merck, catalog number: H9627)
Ammonium iron (III) sulfate (Sigma, catalog number: 221260)
Solutions
2 mM 8-Hydroxyquinoline (see Recipes)
Carnoy’s fluid (see Recipes)
Aceto-haematoxylin-iron alum (see Recipes)
2 mM 8-Hydroxyquinoline
Reagent Final concentration Quantity 8-Hydroxyquinoline 2 mM 29 mg A. bidest 100% 1,000 mL Total 1,000 mL This solution can be kept in the dark at room temperature (RT) for up to one year and can last for up to 100 preparations.
Carnoy’s fluid
Reagent Final concentration Quantity Glacial acetic acid 100% 125 mL Ethanol (absolute) 100% 375 mL Total 500 mL This solution should be prepared immediately before use and can last for up to 50 preparations.
Aceto-haematoxylin-iron alum
Reagent Final concentration Quantity Acetic acid 45% 100 mL Haematoxylin 0.4% 400 mg Ammonium iron (III) sulfate 0.1% 100 mg Total 100 mL This solution can be kept at 4 °C for up to half a year and can last for up to 300 preparations.
Equipment
Gas burner
Fridge (4 °C)
Growth chamber (Panasonic, MLR-352-PE equipped with 2 Panasonic FL40SS·ENW/37 fluorescent tubes)
Light microscope [Zeiss Axiovert 200M microscope equipped with a 100×, 1.3 NA objective lens (Carl Zeiss AG)] with a Zeiss high-resolution AxioCam HRm Rev.3 camera
Glassware: 250–500 mL Erlenmeyer flask (Analyticsshop.com, ID1121226361), 10 mL glass vials (Merck, 27151), 10 mL test tubes (Merck, Z741001), culture dish (Analyticsshop.com, ø 100 mm), glass jar (for storage)
Glass Pasteur pipettes (Analytics Shop.com, BR747715)
LLG-Syringe filters, CA, 0.20 m, ø 13 mm (Lab logistics Group, 14140027207)
1 mL Syringe, Omnifix®-F (Bio-apo.at, 00569881)
2 mL tubes
Lint-free paper
Metal rack
Microscopic slides and coverslips
Wooden clip
Pair of fine-pointed tweezers
Liquid nitrogen container with lid
Leather gloves
Long tongs
Spray skirt
Safety goggles
Stopwatch or timer
Software
Helicon Focus (HeliconSoft Ltd.)
ImageJ (1.53v)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Rittmeier, N. and Holzinger, A. (2023). Improved Methods for Acetocarmine and Haematoxylin Staining to Visualize Chromosomes in the Filamentous Green Alga Zygnema (Charophyta). Bio-protocol 13(16): e4768. DOI: 10.21769/BioProtoc.4768.
分类
植物科学 > 植物细胞生物学 > 细胞成像
细胞生物学 > 细胞结构 > 染色体
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













