- EN - English
- CN - 中文
A Novel Method for Measuring Mitochondrial Respiratory Parameters in Wheat Paleae (Paleae Superior) Using the XF24 Analyzer
使用XF24分析仪测定小麦线粒体呼吸参数的新方法
发布: 2023年08月05日第13卷第15期 DOI: 10.21769/BioProtoc.4767 浏览次数: 1358
评审: Samik BhattacharyaNishanth SekarPriyanka Das
Abstract
Understanding the influence of secondary metabolites from fungi on the mitochondria of the host plant during infection is of great importance for the knowledge of fungus–plant interactions in general; it could help generate resistant plants in the future and in the development of specifically acting plant protection products. For this purpose, it must first be possible to record the mitochondrial parameters in the host plant. As of the date of this protocol, no measurements of mitochondrial respiration parameters have been performed in wheat paleae. The protocol shown here describes the measurements using the XF24 analyzer, which measures the rate of oxygen consumption in the sample by changes in the fluorescence of solid-state fluorophores. This procedure covers the preparation of samples for the XF24 analyzer and the measurement of mitochondrial parameters by adding specific mitochondrial inhibitors. It also shows the necessary approach and steps to be followed to obtain reliable, reproducible results. This is a robust protocol that allows the analysis of mitochondrial respiration directly in the wheat paleae. It demonstrates an important add-on method to existing screenings and also offers the possibility to test the effects of early infection of plants by harmful fungi (e.g., Fusarium graminearum) on mitochondrial respiration parameters.
Key features
• This protocol offers the possibility of testing the effects of early infection of plants by pathogens on mitochondrial respiration parameters.
• This protocol requires a Seahorse XF24 Flux Analyzer with Islet Capture Microplates and the Seahorse Capture Screen Insert Tool.
Graphical overview
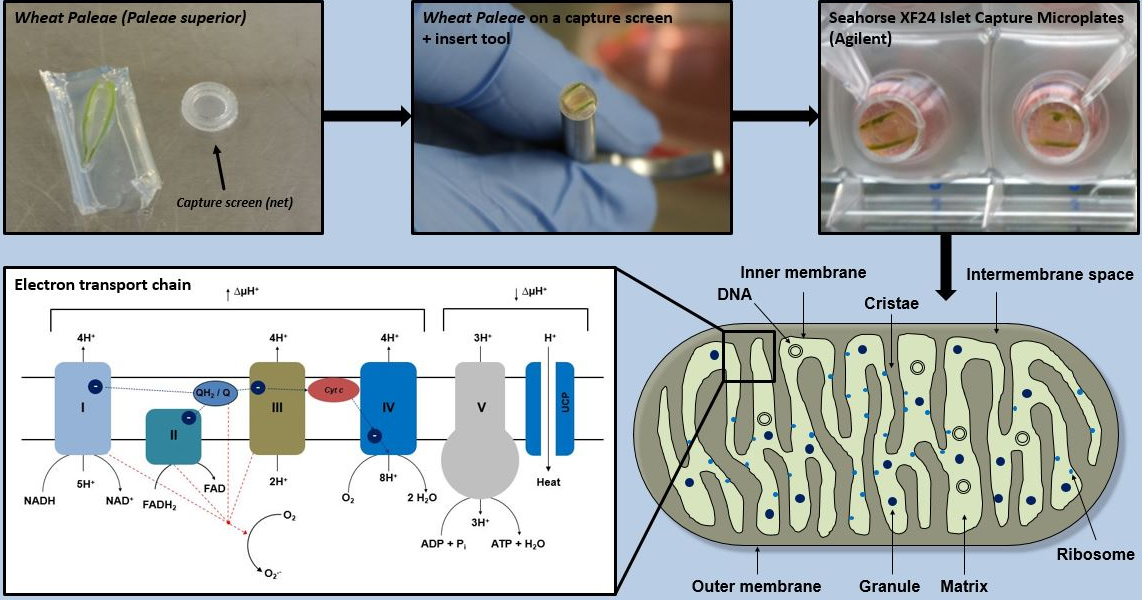
Background
The filamentous ascomycete Fusarium graminearum is the main pathogen causing Fusarium head blight (FHB) in wheat (McMullen et al., 1997). The consequences of host plant infection lead to significant quantitative and economic losses (Goswami and Kistler, 2004), as the infection results in incompletely formed grains. During infection, Fusarium graminearum produces a variety of secondary metabolites, low-molecular-weight molecules that are generally not necessary for the growth or developmental processes of the fungus but result in contamination of the grain with harmful mycotoxins (Shwab and Keller, 2008). Contaminated grain thus becomes unusable as food for humans and animals.
One of these mycotoxins is butenolide, which was first isolated in 1967 and shown to be toxic to mitochondrial respiration in rat cardiac muscle tissue (Wang et al., 2009). Further studies revealed that mitochondria of muscle tissue are affected. This includes swelling, disruption of the double membrane, and disruption of mitochondrial respiration (Wang et al., 2007 and 2009; Pei et al., 2013). Whether plant mitochondria are also affected by butenolide has not yet been investigated in detail. However, the impairment of animal mitochondria by butenolide could be an indication of a possible effect of this metabolite on plant mitochondria. Therefore, understanding the function of these substances on mitochondria during infection is of great importance for the knowledge of fungus–plant interaction in general and should help to produce resistant plants in the future.
Because the measurement of mitochondrial respiration in cells from plant cultures may not fully reflect the real situation, we describe here a method for measuring mitochondrial respiration parameters in wheat paleae (paleae superior) using an XF24 Extracellular Flux Analyzer. To date, no measurements of mitochondrial respiratory parameters have been performed in wheat paleae. The XF24 analyzer is a respirometer based on a multi-well plate that measures oxygen consumption rate (OCR) by changes in the fluorescence of solid-state fluorophores (Gerencser et al., 2009). To obtain a mitochondrial respiration profile, mitochondrial agents are automatically injected through various ports during testing (Divakaruni et al., 2014). This procedure involves preparation of samples for the XF24 analyzer and the measurement of mitochondrial parameters by addition of specific mitochondrial inhibitors.
The analysis of mitochondrial respiration in these samples is an important addition to existing studies and also offers the possibility to test the effects of early infection of plants with harmful fungi on mitochondrial respiration parameters. This could contribute to the development of resistant plants as well as specifically acting plant protection products. In addition to questions on resistance to fungal attack, this protocol can be used to investigate questions relating to mitochondrial activity in wheat in more detail or to use mitochondrial parameters as a supplement to classical phenotyping.
Materials and reagents
Centrifuge tubes 15 and 50 mL (Sarstedt, catalog numbers: 62.554.001, 62.547.254)
Petri dishes (Sarstedt, catalog number: 83.3902)
Pipette tips 10, 20, 200, and 1,000 μL (Sarstedt, catalog numbers: 70.1130, 70.116, 70.760.002, 70.762)
Sterile filter 0.2 μm (Sarstedt, catalog number: 83.1826.001)
Wheat paleae (kept on 1.6% agarose gel at 4 °C for a maximum of 36 h) (prevention of dehydration during transport)
KCl (Carl Roth, catalog number: 6781.3)
KH2PO4 (Carl Roth, catalog number: 3904.2)
Na2HPO4·12H2O (Carl Roth, catalog number: N350.1)
NaCl (Carl Roth, catalog number: 3957.3)
NaOH (Carl Roth, catalog number: 6771.3)
Seahorse XF calibrant solution (Agilent Technologies, catalog number: 100840-000)
Seahorse XF Cell Mito Stress Test kit [oligomycin, FCCP (Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone), and rotenone/antimycin A] (Agilent Technologies, catalog number: 103015-100); store at -20 °C
Agarose standard (Carl Roth, catalog number: 3810.3)
Dulbecco’s modified Eagle’s medium, high glucose (Sigma-Aldrich, catalog number: D7777-10L), storage at 4 °C
10 M NaOH (for adjustment of pH values) (see Recipes)
1.6% agarose gel (see Recipes)
1× PBS (see Recipes)
XF assay medium (see Recipes)
Equipment
Scalpel, stainless steel (Carl Roth, catalog number: T997.1)
Seahorse Capture Screen Insert Tool (Agilent Technologies, catalog number: 101135-100)
Seahorse XF24 Islet Capture FluxPak containing sensor cartridges and Islet Capture Microplates (Agilent Technologies, catalog number: 101174-100)
Tweezers, stainless steel (Carl Roth, catalog number: 2687.1)
Incubator at 37 °C without CO2 (GFL, catalog number: 4010)
pH Meter FiveEasyTM F20 (Mettler Toledo, catalog number: 30266626)
Pipettes, Eppendorf Research® Plus 10, 20, 200, and 1,000 μL (Eppendorf, catalog numbers: 3123000020, 3123000039, 3123000055, 3123000063)
Seahorse XF24 Flux Analyzer (Agilent Technologies, model: XF24, catalog number: 100737-100)
Software and datasets
Seahorse XF24 Flux Analyzer Software (instrument software) (Agilent Technologies, Version 1.8.1.1)
Wave (analysis software) (Agilent Technologies, Version 2.6.1)
GraphPad Prism (statistical software) (GraphPad Software, Inc., Version 9.5.1)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Schniertshauer, D. and Bergemann, J. (2023). A Novel Method for Measuring Mitochondrial Respiratory Parameters in Wheat Paleae (Paleae Superior) Using the XF24 Analyzer. Bio-protocol 13(15): e4767. DOI: 10.21769/BioProtoc.4767.
分类
植物科学 > 植物生理学 > 新陈代谢
细胞生物学 > 基于细胞的分析方法 > 线粒体呼吸
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












