- EN - English
- CN - 中文
Establishment of Human PD-1/PD-L1 Blockade Assay Based on Surface Plasmon Resonance (SPR) Biosensor
基于表面等离子体共振(SPR)生物传感器的人PD-1/PD-L1阻断检测方法的建立
(*contributed equally to this work, § Technical contact) 发布: 2023年08月05日第13卷第15期 DOI: 10.21769/BioProtoc.4765 浏览次数: 2842
评审: Kazem NouriToshitsugu FujitaAnonymous reviewer(s)

相关实验方案

膜蛋白–配体相互作用研究的计算流程:以含 α5 亚基的 GABA(A) 受体为例
Syarifah Maisarah Sayed Mohamad [...] Ahmad Tarmizi Che Has
2025年11月20日 2123 阅读
Abstract
Blockade of the programmed cell death protein 1 (PD-1)/PD-ligand 1 (PD-L1) axis is a promising strategy for cancer immunotherapy. Although antibody-based PD-1/PD-L1 inhibitors have shown remarkable results in clinical cancer studies, their inherent limitations underscore the significance of developing novel PD-1/PD-L1 inhibitors. Small molecule inhibitors have several advantages over antibody-based inhibitors, including favorable tumor penetration and oral bioavailability, fewer side effects, easier administration, preferred biological half-life, and lower cost. However, small molecule inhibitors that directly target the PD-1/PD-L1 interaction are still in the early development stage, partially due to the lack of reliable biophysical assays. Herein, we present a novel PD-1/PD-L1 blockade assay using a surface plasmon resonance (SPR)-based technique. This blockade assay immobilizes human PD-1 on a sensor chip, which interacts with PD-L1 inhibitors or negative PD-L1 binders with human PD-L1 protein at a range of molecular ratios. The binding kinetics of PD-L1 to PD-1 and the blockade rates of small molecules were determined. Compared to other techniques such as PD-1/PD-L1 pair enzyme-linked immunosorbent assay (ELISA) and AlphaLISA immunoassays, our SPR-based method offers real-time and label-free detection with advantages including shorter experimental runs and smaller sample quantity requirements.
Key features
• A SPR protocol screens compounds for their capacity to block the PD-1/PD-L1 interaction.
• Validation of PD-1/PD-L1 interaction, followed by assessing blockade effects with known inhibitors BMS-1166 and BMS-202, and a negative control NO-Losartan A.
• Analysis of percentage blockade of PD-1/PD-L1 of the samples to obtain the IC50.
• Broad applications in the discovery of small molecule–based PD-1/PD-L1 inhibitors for cancer immunotherapy.
Graphical overview
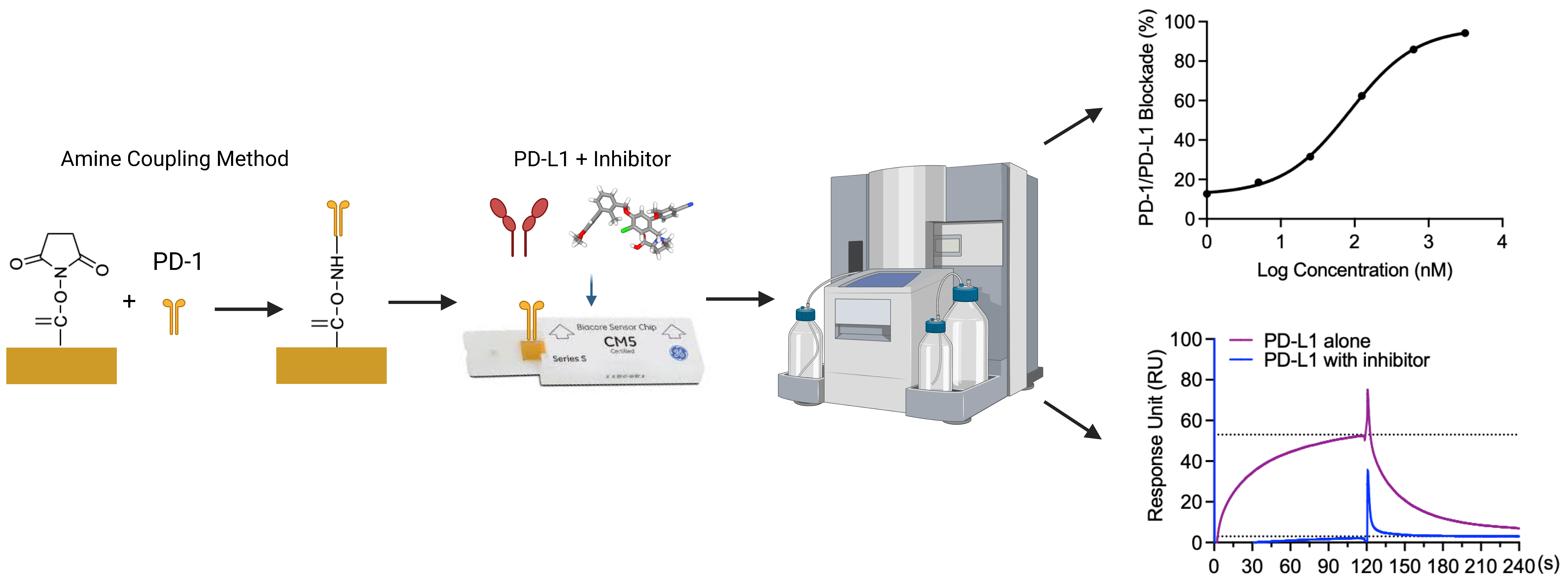
Background
Cancer is a global health burden resulting in high healthcare costs. Therefore, the search for effective therapeutics is of continued scientific interest. Recently, cancer immunotherapy, a strategy that utilizes the host’s own immune system to fight tumors, has become an effective treatment of cancers (Makuku et al., 2021). In cancer, the tumor cell microenvironment acts to inhibit immune checkpoints, which normally function to prevent uncontrolled proliferation (He and Xu, 2020). Programmed cell death protein 1 (PD-1), an immune checkpoint expressed by several types of immune cells, dampens the immune system upon programmed cell death ligand-1 (PD-L1) binding (Makuku et al., 2021). The interaction of PD-1/PD-L1 leads to the inhibition of phosphorylation of the T-cell-receptor (TCR) signaling intermediate, which terminates the TCR signaling cascade (Keir et al., 2008; Fife et al., 2009). Two signaling motifs in the cytoplasmic tail of PD-1 are the intracellular immunoreceptor tyrosine-based switch motif (ITSM) and the immunoreceptor tyrosine-based inhibitory motif. Upon PD-L1 binding to PD-1, ITSM is phosphorylated and recruits Src homology 2-containing tyrosine phosphatase, thereby inhibiting the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway (Barclay et al., 2018; Ai et al., 2020). PI3K/Akt signaling pathway blockage further downregulates the mechanistic targets of rapamycin and inhibits protein synthesis and cell growth. PI3K/Akt signaling pathway blockage also inhibits the degradation of transcription factor FoxO1, which enhances the expression of PD-1 (Barclay et al., 2018; Ai et al., 2020).
The recognition of the PD-1 protein on the membrane of T cells by tumor cells results in the upregulation of PD-L1 (J. Liu et al., 2021). High expression of PD-L1 is one characteristic observed in many types of tumors including melanoma, lung cancer, and breast cancer (Mu et al., 2011; Fusi et al., 2015; Aguilar et al., 2019). PD-1/PD-L1 binding results in T-cell apoptosis (J. Liu et al., 2021). Blockade of the PD-1/PD-L1 axis results in tumor suppression due to interference between the tumor cell and the T cell (C. Liu et al., 2021; Makuku et al., 2021). Numerous studies have demonstrated that the blockage of PD-L1 or PD-1 is one of the most promising approaches for cancer immunotherapy (Zitvogel and Kroemer, 2012; Wu et al., 2018; Salmaninejad et al., 2019). Blocking the interactions of PD-L1 and PD-1 shuts off the inhibitory signaling pathways for T cells, reactivates the T cell–mediated anti-tumor responses by promoting T-cell proliferation, and enhances effector T-cell function (Salmaninejad et al., 2019; Tang and Zheng., 2018). Clinical data have demonstrated that the blockade of PD-1 or PD-L1 can boost T cell–mediated antitumor responses, generates durable clinical responses, and prolongs patient survival time (Ohaegbulam et al., 2015; Alsaab et al., 2017). Monoclonal antibodies against PD-1 (Pembrolizumab, Nivolumab, and Cemiplimab) or PD-L1 (Atezolizumab, Avelumab, and Durvalumab) have been approved by FDA for the treatment of a series of malignancies including breast cancer, bladder cancer, colorectal cancer, lung cancer, hepatoma, and melanoma (Massard et al., 2016; Kim, 2017; Xin Yu et al., 2020). Although these monoclonal antibodies demonstrated promising results with high clinical efficacy and immune-related adverse effects, immunogenicity and high costs are still major limitations of antibody-based immune checkpoint inhibitors (Hamanishi et al., 2015; Alsaab et al., 2017; Zinzani et al., 2017; Akinleye and Rasool et al., 2019). Alternatively, small-molecule inhibitors can overcome these advantages due to better tumor penetration and oral availability (Zhan et al., 2016).
Therefore, the discovery of small molecule inhibitors blocking the PD-1/PD-L1 interaction is a promising cancer therapy approach. Our group reported the evaluation of the PD-1/PD-L1 blockade (using a pair-ELISA technique) and the binding of compounds to either PD-1 or PD-L1 (Li et al., 2022). However, this method is not efficient for large-scale screenings of small molecule libraries for PD-1/PD-L1 inhibitors. Therefore, we developed a surface plasmon resonance (SPR)-based PD-1/PD-L1 blockade screening approach utilizing immobilized PD-1 (on the chip), PD-L1 (in solution), and known inhibitors (i.e., BMS-1166 or BMS-202, in solution). To exclude potential false positives, we included a negative PD-L1 inhibitor (NO-Losartan A) possessing a biphenyl group—a structural feature shared with the BMS-1166 and the BMS-202 compounds that were investigated together in this study. Notably, the SPR technique is a valuable complementary method to ELISA immunoassays that can also be used for the optimization of ELISA-based assays (Vaisocherová et al., 2009). SPR is an optical biosensor technology that employs the evanescent wave phenomenon to detect changes in the refractive index of a biosensor (Pattnaik, 2005). A light source illuminates the biosensor and prism, and as the analyte flows through the channel and binds to the target protein, the refractive index of the biosensor undergoes a shift. This interaction between analyte and protein is monitored in real-time, enabling precise measurement of the amount of bound protein as well as the rates of association and dissociation. The SPR assay has unique advantages over the ELISA-type assay. Rather than merely providing an endpoint, the SPR assay monitors the kinetics associated with the PD-1/PD-L1 blockade of small molecules in real time. We acknowledge that ELISA-type assays are more widely accessible and adaptable to different laboratory settings, and we recognize that our SPR assay requires specialized instrumentation and expertise, which may not be available in all laboratories. Furthermore, given that this blockade assay is solely based on in vitro experiments, it is imperative to perform functional assays and in vivo validation to confirm the potential of compounds exhibiting blockade effects against PD-1/PD-L1. However, we believe that this SPR-based protocol, which provides sufficient details, can facilitate the screening process of small molecule inhibitors that block the PD-1/PD-L1 interaction at a large scale.
In the present study, we utilized an SPR-based assay to determine the IC50 values of BMS-1166 and BMS-202, which were measured at 85.4 and 654.4 nM, respectively. BMS-1166 has been previously characterized with an IC50 value of 1.4 and 276 nM by homogeneous time-resolved fluorescence (HTRF) and cell-based assays (Jurkat cells expressing PD-1 in co-culture with CHO cells expressing PD-L1), respectively (Guzik et al., 2017). Previous investigations have reported IC50 values of BMS-202 at 18 and 96 nM utilizing different assays, including cell-based and HTRF approaches (Surmiak et al., 2021). Our results are comparable with previous findings and confirm the reliability and reproducibility of our SPR-based protocol.
Materials and reagents
Biological materials
Human PD-L1/B7-H1 protein, Fc Tag (ACROBiosystems, catalog number: D1-H5258)
Human PD-1/PDCD1 protein, Fc Tag, low endotoxin (ACROBiosystems, catalog number: PD1-H5257)
Reagents
BMS-1166 (Med Chem Express, catalog number: HY-102011)
BMS-202 (Med Chem Express, catalog number: HY-19745)
Amine Coupling kit [ethanolamine hydrochloride, dimethylaminopropyl-N’ethylcarbodiimide N-3-hydrochloride (EDC), and N-hydroxy succinimide (NDC)] (Global Life Sciences Solutions, Cytiva, catalog number: BR100050)
NO-Losartan A (Cayman Chemical Company, catalog number: Cay10006456)
Solutions
HBS-EP+ buffer 10× (Global Life Sciences Solutions, Cytiva, catalog number: BR100826)
Glycine 1.5 (Global Life Sciences Solutions, Cytiva, catalog number: BR100354)
Dimethyl sulfoxide (DMSO), anhydrous ≥ 99.9% (Sigma-Aldrich, catalog number: 276855)
DNase-free water (Fisher Scientific, catalog number: 188506)
Acetate 5.0 (Global Life Sciences Solutions, Cytiva, catalog numbers: BR100350, BR100351)
NaOH 50 mM (Global Life Sciences Solutions, Cytiva, catalog number: 100358)
PD-1 or PD-L1 protein solution, 500 μg/mL (PD-L1 protein solution equals to 2,000 nM) (see Recipes)
HBS-EP+ running buffer (250 mL Fisherbrand glass bottle) (see Recipes)
HBS-EP+ running buffer + 0.01% DMSO (250 mL Fisherbrand glass bottle) (see Recipes)
Recipes
PD-1 or PD-L1 protein solution, 500 μg/mL (PD-L1 protein solution equals to 2,000 nM)
Add 200 μL of DNase-free water to 100 μg of PD-1 or PD-L1 protein. (To prevent nucleic acid contamination, it is recommended to use DNase-free water for the preparation of a long-term stock solution of PD-1 or PD-L1 proteins. However, for assays utilizing fresh protein solutions, Milli-Q water is sufficient.)
HBS-EP+ running buffer (250 mL Fisherbrand glass bottle)
Add 20 mL of the HBS-EP+ buffer 10× to 180 mL of Milli-Q water.
HBS-EP+ running buffer + 0.01% DMSO (250 mL Fisherbrand glass bottle)
Add 25 mL of the HBS-EP+ buffer 10× to 224.975 mL of Milli-Q water.
Add 25 μL DMSO.
Laboratory supplies
Pipettes (ErgoOne Single Channel Pipette 2.5, 10, 200, 1,000 μL; USA Scientific, catalog numbers: 7100-0125, 7100-0510, 7100-2200, 7110-1000)
Pipette Tips (TipOne 10, 200, 1,000 μL; USA Scientific, catalog numbers: 1111-3800, 1110-1800, 1111-2821)
SCI-Fill motorized pipette filler (Scilogex, catalog number: 740200029999)
Serological pipettes 10 and 50 mL (Thermo ScientificTMNuncTM, catalog numbers: 02-923-204, 02-923-206)
96-well polystyrene microplates (Global Life Sciences Solutions, Cytiva, catalog number: BR100503)
Microplate foil, 96-well (Global Life Sciences Solutions, Cytiva, catalog number: 28975816)
Plastic vials 7 mm (Global Life Sciences Solutions, Cytiva, catalog number: BR100212)
Rubber caps, type 3 (Global Life Sciences Solutions, Cytiva, catalog number: BR100502)
Series S Sensor Chip CM5 (Global Life Sciences Solutions, Cytiva, catalog number: BR100530)
Fisherbrand reusable glass media bottles with cap 250 mL (Fisher Scientific, catalog number: FB800100)
Microcentrifuge tube 1.5 mL non-sterile (Cell Treat, Wilkem Scientific, catalog number: LCEL229441)
PCR tubes individual 0.2 mL flat cap (PureAmp, Wilkem Scientific, catalog number: LMTP3030)
Equipment
Biacore T200 SPR (Global Life Sciences Solutions, Cytiva, catalog number: 28975001)
Software and datasets
Biacore T200 analysis software (BIAevaluation version 4.1)
GraphPad Prism 9.1.2 (https://www.graphpad.com/updates/prism-912-release-notes)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Puopolo, T., Li, H., Gutkowski, J., Cai, A., Seeram, N. P., Ma, H. and Liu, C. (2023). Establishment of Human PD-1/PD-L1 Blockade Assay Based on Surface Plasmon Resonance (SPR) Biosensor. Bio-protocol 13(15): e4765. DOI: 10.21769/BioProtoc.4765.
分类
生物化学 > 蛋白质 > 相互作用 > 蛋白质-配体相互作用
药物发现 > 药物筛选
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










