- EN - English
- CN - 中文
Large-scale Purification of Type III Toxin-antitoxin Ribonucleoprotein Complex and its Components from Escherichia coli for Biophysical Studies
从大肠杆菌中大规模纯化III型毒素-抗毒素核糖蛋白复合物及其成分,用于生物物理学研究
(*contributed equally to this work) 发布: 2023年07月05日第13卷第13期 DOI: 10.21769/BioProtoc.4763 浏览次数: 2174
评审: Alba BlesaValentine V TrotterAnonymous reviewer(s)
Abstract
Toxin–antitoxin (TA) systems are widespread bacterial immune systems that confer protection against various environmental stresses. TA systems have been classified into eight types (I–VIII) based on the nature and mechanism of action of the antitoxin. Type III TA systems consist of a noncoding RNA antitoxin and a protein toxin, forming a ribonucleoprotein (RNP) TA complex that plays crucial roles in phage defence in bacteria. Type III TA systems are present in the human gut microbiome and several pathogenic bacteria and, therefore, could be exploited for a novel antibacterial strategy. Due to the inherent toxicity of the toxin for E. coli, it is challenging to overexpress and purify free toxins from E. coli expression systems. Therefore, protein toxin is typically co-expressed and co-purified with antitoxin RNA as an RNP complex from E. coli for structural and biophysical studies. Here, we have optimized the co-expression and purification method for ToxIN type III TA complexes from E. coli that results in the purification of TA RNP complex and, often, free antitoxin RNA and free active toxin in quantities required for the biophysical and structural studies. This protocol can also be adapted to purify isotopically labelled (e.g., uniformly 15N- or 13C-labelled) free toxin proteins, free antitoxin RNAs, and TA RNPs, which can be studied using multidimensional nuclear magnetic resonance (NMR) spectroscopy methods.
Key features
• Detailed protocol for the large-scale purification of ToxIN type III toxin–antitoxin complexes from E. coli.
• The optimized protocol results in obtaining milligrams of TA RNP complex, free toxin, and free antitoxin RNA.
• Commercially available plasmid vectors and chemicals are used to complete the protocol in five days after obtaining the required DNA clones.
• The purified TA complex, toxin protein, and antitoxin RNA are used for biophysical experiments such as NMR, ITC, and X-ray crystallography.
Graphical overview
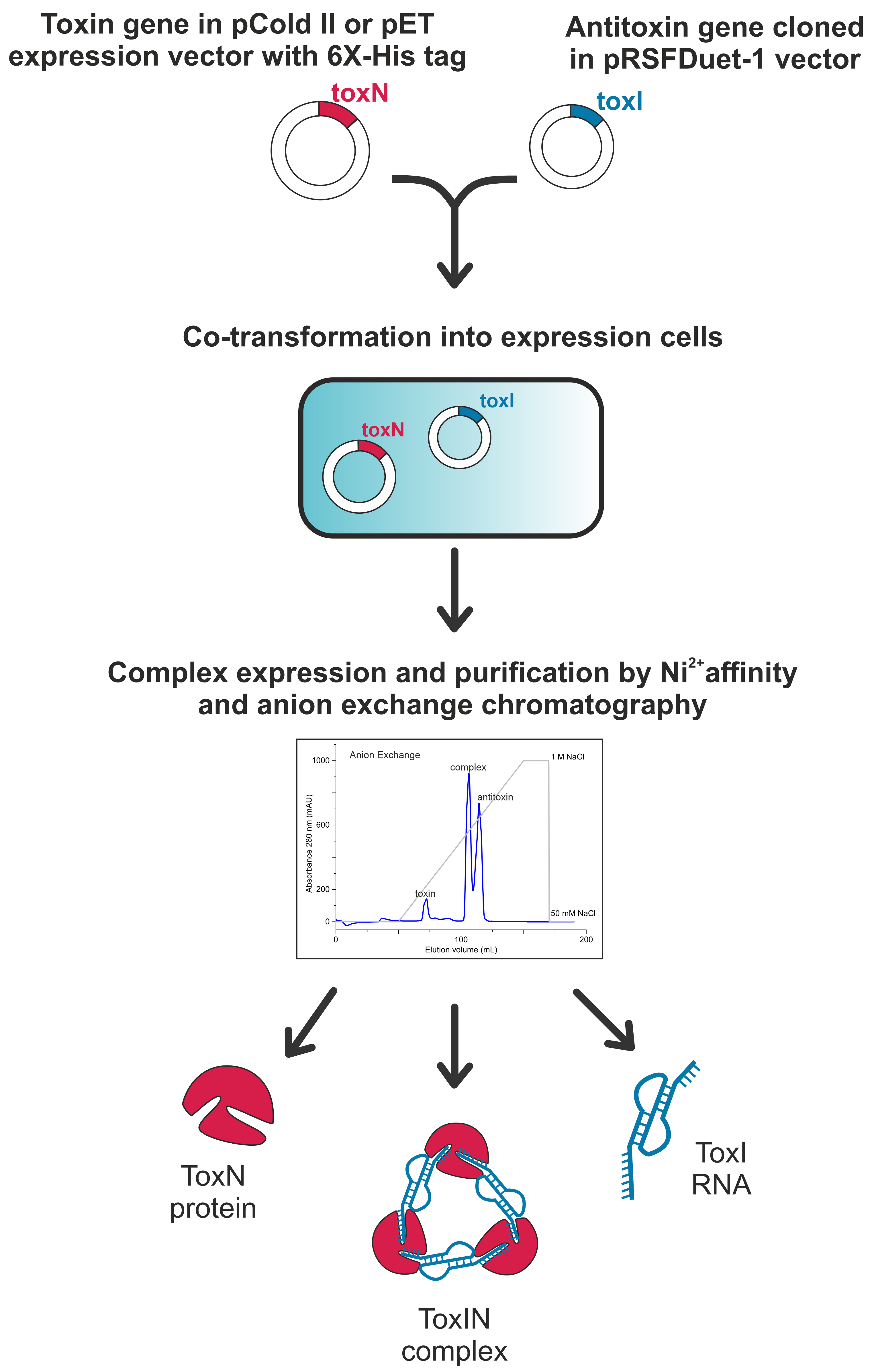
Background
Type III toxin–antitoxin (TA) systems are genetic modules found in several bacteria, consisting of a protein toxin and a noncoding RNA antitoxin (Fineran et al., 2009). Antitoxin RNA repeats directly bind to the protein toxin, inhibiting its activity by forming a TA ribonucleoprotein (RNP) complex under homeostatic conditions. The primary functions of type III TA systems are attributed to the phage inhibition mechanism in bacteria (Song and Wood, 2020; LeRoux and Laub, 2022; Lin et al., 2023). Type III TA systems are widespread and present in several pathogenic bacteria, including Staphylococcus aureus, Yersinia pseudotuberculosis, and Fusobacterium nucleatum (Blower et al., 2012), and several bacteria in the gut microbiome. Thus, understanding the structure and assembly of type III TA systems could help in devising novel antibacterial strategies by targeting these systems to free the toxins in the pathogenic bacteria, resulting in bacterial growth arrest or death.
The type III TA systems are classified into three families: ToxIN, CptIN, and TenpIN, based on toxin sequence homology (Blower et al., 2012). Among these classes, the ToxIN family (here, protein toxin and antitoxin RNA are called ToxN and ToxI, respectively) is the most studied in terms of its structure, assembly, and functions (Blower et al., 2011; Short et al., 2012; Guegler and Laub, 2021; Manikandan et al., 2022). In a recent study, we classified E. coli ToxIN TA systems into five clusters based on the sequence analysis of the toxin proteins (Manikandan et al., 2022). The structural and functional analysis of a CptIN TA complex from Eubacterium rectale has also been reported (Rao et al., 2015).
It is often difficult to purify free toxins from E. coli due to their inherent toxicity to the bacteria. To circumvent this issue, toxin and antitoxin are often co-expressed and co-purified as non-toxic TA complexes. This is followed by denaturing the TA complex (e.g., in the presence of 6 M guanidinium hydrochloride or 8 M urea) to separate the toxin and antitoxin. The free denatured toxin can then be refolded back to its natural form. However, in vitro refolding of proteins is not always successful. Alternatively, in some cases, active-site toxin mutants have been generated, which can be expressed in E. coli and purified (Samson et al., 2013). While several experiments can be performed using the catalytic mutant protein, a wild-type active protein is often still required to understand the toxins’ mechanism of action.
Here, by adapting the methods published earlier (Blower et al., 2011; Short et al., 2012; Rao et al., 2015; Manikandan et al., 2022), we have optimized a protocol to co-express and co-purify the ToxIN complexes and their components from three clusters of ToxIN family from E. coli (Manikandan et al., 2022) for their biophysical and structural investigation [using X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy]. The method described here yields TA RNP complex, free protein toxin, and free antitoxin RNA of the ToxIN system with an approximate molar ratio of 3:2:2 (with an approximate yield of 7, 3, and 1.5 mg, respectively, from one litre of LB media). The availability of a robust purification method could potentially break the barrier between the discovery of new type III TA systems and their subsequent biophysical characterization to understand their mechanism of action.
Materials and reagents
Biological materials
E. coli BL21(DE3) competent cells (prepared in the lab following a standard protocol) (Sambrook and Russell, 2006)
E. coli DH5α competent cells (prepared in the lab following a standard protocol)
pColdTM II vector (Takara Bio, catalog number: 3362)
pET-21a(+) (Novagen, catalog number: 69740-3)
pETDuetTM-1 vector (Novagen, catalog number: 71146-3)
pRSFDuetTM-1 vector (Novagen, catalog number: 71341-3)
Reagents, chemicals, and kits
Acetic acid (glacial) (Sisco Research Laboratories, catalog number: 85801, CAS number: 64-19-7)
Acrylamide:Bis-acrylamide (19:1) for electrophoresis, 40% solution (Fischer BioReagentsTM, catalog number: BP1406-1, CAS number: 79-06-1), store at 4–8 °C
Agarose low EEO (Sisco Research Laboratories, catalog number: 36601, CAS number: 9012-36-6)
Ammonium persulfate (APS) (Sisco Research Laboratories, catalog number: 65553, CAS number: 7727-54-0)
Ampicillin (Sisco Research Laboratories, catalog number: 61314, CAS number: 69-52-3), store at 2–8 °C
Bradford reagent (Sigma, catalog number: B6916-500 mL), store at 2–8 °C
Bromophenol Blue indicator (Sisco Research Laboratories, catalog number: 11458, CAS number: 115-39-9)
Coomassie brilliant blue (Sisco Research Laboratories, catalog number: 93473, CAS number: 6104-58-1)
D/L-Dithiothreitol (DTT) (Sisco Research Laboratories, catalog number: 17315, CAS number: 3483-12-3), store at 0–4 °C
dNTPs (New England Biolabs, catalog number: N0447S), store at -20 °C
DpnI (New England Biolabs, catalog number: R0176), store at -20 °C
Ethanol (Changshu Hongsheng Fine Chemicals, analytical grade, UN No: 1170)
Ethylenediaminetetraacetic acid (EDTA) (Sisco Research Laboratories, catalog number: 50027, CAS number: 6381-92-6)
Formamide (Sisco Research Laboratories, catalog number: 71714 (062930), CAS number: 75-12-7)
Gel extraction kit (QIAquick®, Qiagen, catalog number: 28704)
Glycerol (Sisco Research Laboratories, catalog number: 62417, CAS number: 56-81-5)
Glycine (Sisco Research Laboratories, catalog number: 64072, CAS number: 56-40-6)
Hydrochloric acid (Sisco Research Laboratories, catalog number: 65955, CAS number: 7647-01-0)
Imidazole (Sisco Research Laboratories, catalog number: 61510-500G)
Isopropyl β-d-1-thiogalactopyranoside (IPTG) (Sisco Research Laboratories, catalog number: 67208, CAS number: 367-93-1), store at 0–4 °C
Kanamycin (Sisco Research Laboratories, catalog number: 99311, CAS number: 25389-94-0), store at 2–8 °C
Luria Bertani agar (LA), Miller (Himedia, catalog number: M1151-500G)
Luria broth (LB) (Himedia, catalog number: M575-500G)
Methanol for HPLC (SD Fine Chemical Limited, catalog number: 25217 L25)
NaCl (Sisco Research Laboratories, catalog number: 41721, CAS number: 7647-14-5)
NcoI (New England Biolabs, catalog number: R3193S), store at -20 °C
NdeI (New England Biolabs, catalog number: R0111S), store at -20 °C
PCR purification kit (Qiagen, QIAquick® PCR purification kit, catalog number: 28104)
Phusion High-Fidelity DNA polymerase (New England Biolabs, catalog number: M0530S), store at -20 °C
Plasmid extraction kit (QIAprep® spin Miniprep kit, Qiagen, catalog number: 27106)
Protease inhibitor cocktail (Roche, catalog number: 04693159001), store at 2–8 °C
Quick calf intestinal phosphatase (CIP) (New England Biolabs, catalog number: M0525S), store at -20 °C
Sodium dodecyl sulphate (SDS) (Sisco Research Laboratories, catalog number: 32096, CAS number: 151-21-3)
T4 DNA ligase (New England Biolabs, catalog number: M0202S), store at -20 °C
Tetramethylethylenediamine (TEMED) (Spectrochem private limited, catalog number: 012017), store at 2–8 °C
Toluidine Blue (Sisco Research Laboratories, catalog number: 22134, CAS number: 92-32-9)
Tris (Sisco Research Laboratories, catalog number: 71033-500G)
Urea (Sisco Research Laboratories, catalog number: 21113, CAS number: 57-13-6)
XhoI (New England Biolabs, catalog number: R0145S), store at -20 °C
β-Mercaptoethanol (Sisco Research Laboratories, catalog number: 83759, CAS number: 60-24-2)
Plastic and other materials
Centrifuge bottle 500 mL (Tarsons, catalog number: 544020)
Centrifuge tubes 15 mL (Tarsons, SpinwinTM Tube Conical bottom, catalog number: 520060)
Centrifuge tubes 50 mL (Tarsons, catalog number: 520061)
Membrane filters (0.22 μm) (Merck Life Science private limited, catalog number: GVWP04700)
Membrane filters (0.45 μm) (Merck Life Science private limited, catalog number: HVLP04700)
Microcentrifuge tubes 1.5 mL (Tarsons, catalog number: 500010)
Microcentrifuge tubes 2 mL (Tarsons, catalog number: 500020)
Oakridge centrifuge tubes (50 mL) (Thermo Scientific, catalog number: 3115-0050)
PCR tubes 0.2 mL flat cap (Tarsons, catalog number: 510051)
SnakeSkin dialysis bag 3.5 kDa cut off (dialysis bag) (Thermo Scientific, catalog number: 88242), store at 2–8 °C
Syringe filters (0.22 μm) (Sartorius Minisart®, catalog number: S6534-FMOSK)
Syringe filters (0.45 μm) (Sartorius Minisart®, catalog number: S6555-FMOSK)
Syringes 10 mL (Hindustan Syringes and Medical Devices Limited, Dispo Van, India)
Syringes 20 mL (Hindustan Syringes and Medical Devices Limited, Dispo Van, India)
PCR reaction mixture for all the amplifications (see Recipes)
Double digestion of vector and insert using restriction enzymes (see Recipes)
Ligation reaction of vector and insert (see Recipes)
Lysis buffer (see Recipes)
Wash buffer (see Recipes)
Elution buffer (see Recipes)
Final wash buffer (see Recipes)
Dialysis buffer (see Recipes)
Ion exchange chromatography buffers (see Recipes)
Size exclusion chromatography buffer (see Recipes)
Separating gel for 12% SDS-PAGE (see Recipes)
Stacking gel for SDS-PAGE (see Recipes)
5× loading dye for SDS-PAGE (see Recipes)
Staining solution for SDS-PAGE (see Recipes)
10× running buffer for SDS-PAGE (see Recipes)
SDS-PAGE destaining solution (see Recipes)
Urea-acrylamide gel (see Recipes)
Formamide dye (see Recipes)
SDS-loading dye (5×) (see Recipes)
Tris-borate-EDTA (TBE) buffer (see Recipes)
Recipes
PCR reaction mixture for all the amplifications
Reaction component Volume (μL) (per 20 μL reaction) 5× Phusion HF buffer 4 10 mM dNTPs 0.4 10 μM forward primer 1 10 μM reverse primer 1 Template DNA (100 ng/μL) 1 Autoclaved MilliQ H2O 12.4 Phusion® High-Fidelity DNA Polymerase 0.2 Total volume 20 Double digestion of vector and insert using restriction enzymes
Reaction component Volume (μL) (per 50 μL reaction) 10× Cutsmart buffer 5 Enzyme 1 1 Enzyme 2 1 Vector/insert DNA 5 μg/2 μg Autoclaved MilliQ H2O make up to 50 μL Total volume 50 Ligation reaction of vector and insert
Reaction component Volume (μL) (per 20 μL reaction) 10× ligase buffer 2 Digested purified vector (10 ng/μL)
Insert (10 ng/μL)
5
5
T4 DNA ligase
Autoclaved MilliQ H2O
1
7
Total volume 20 Lysis buffer
Reagent Final concentration Amount NaCl (4 M) 300 mM 37.5 mL Tris-HCl (1 M, pH 7.5)
Imidazole (5 M, pH 8.0)
Glycerol (100%)
β-mercaptoethanol (14.28 M)
50 mM
10 mM
10%
2 mM
25 mL
1 mL
50 mL
70 μL
MilliQ H2O n/a 386.43 mL Total n/a 500 mL Wash buffer
Reagent Final concentration Amount Lysis buffer n/a 49.9 mL Imidazole (5 M, pH 8.0) 20 mM 100 μL Total n/a 50 mL Elution buffer
Reagent Final concentration Amount Lysis buffer n/a 24 mL Imidazole (5 M, pH 8.0) 200 mM 1 mL Total n/a 25 mL Final wash buffer
Reagent Final concentration Amount Lysis buffer n/a 18 mL Imidazole (5 M, pH 8.0) 500 mM 2 mL Total n/a 20 mL Dialysis buffer
Reagent Final concentration Amount NaCl (4 M) 50 mM 25 mL Tris-HCl (1 M, pH 7.5) 50 mM 100 mL DTT 1 mM 308.48 mg MilliQ H2O n/a 1,875 mL Total n/a 2 L Ion-exchange chromatography buffers
Buffer A: same as dialysis buffer.
Buffer B:
Reagent Final concentration Amount NaCl (4 M) 1 M 125 mL Tris-HCl (1 M, pH 7.5) 50 mM 25 mL DTT 1 mM 77.12 mg MilliQ H2O n/a 350 mL Total n/a 500 mL Size-exclusion chromatography buffer
Reagent Final concentration Amount NaCl (4 M) 50 mM 6.25 mL Tris-HCl (1 M, pH 7.5) 20 mM 10 mL DTT 1 mM 77.12 mg MilliQ H2O n/a 483.75 mL Total n/a 500 mL Separating gel for 12% SDS-PAGE (prepare solution volume depending on the gel cast size)
Reagent Amount Acrylamide:Bis-acrylamide (19:1) (40% solution) 2.4 mL 1.5 M Tris, pH = 8.8 2 mL 10% SDS solution 80 μL 10% APS solution 80 μL TEMED 8 μL MilliQ H2O 3.432 mL Total 8 mL Stacking gel for SDS-PAGE (prepare solution volume depending on the gel cast size)
Reagent Amount Acrylamide:Bis-acrylamide (19:1) (40% solution) 0.75 mL 1.5 M Tris, pH = 6.8 1.25 mL 10% SDS solution 50 μL 10% APS solution 50 μL TEMED 5 μL MilliQ H2O 2.9 mL Total 5 mL 5× loading dye for SDS-PAGE
Reagent Amount β-Mercaptoethanol 5% Bromophenol Blue 0.02% Glycerol 30% SDS 10% Tris-Cl (pH = 6.8) 250 mM Staining solution for SDS-PAGE
Reagent Amount Coomassie brilliant blue 1 g Methanol 400 mL Acetic acid 100 mL MilliQ H2O 500 mL Total 1 L 10× running buffer for SDS-PAGE
Reagent Amount Tris base 30 g Glycine 144 g SDS 10 g MilliQ H2O Make up to 1L Total 1 L SDS-PAGE destaining solution
Reagent Amount Methanol 40 mL Acetic acid (glacial) 10 mL MilliQ H2O 50 mL Total 100 mL Urea-acrylamide gel (prepare solution volume depending on the gel cast size)
Reagent Concentration Acrylamide:Bis-acrylamide (19:1) 15% Urea 8 M TBE buffer 1× APS 1% TEMED 0.1% MilliQ H2O Make up the volume as per requirement Formamide dye
Prepared using a CSHL protocol for formamide gel-loading buffer (Cold Spring Harb Protoc, 2013)
SDS-loading dye (5×)
Prepared using a CSHL protocol (Cold Spring Harb Protoc, 2008)
Tris-borate-EDTA (TBE) buffer
Prepared according to a CSHL protocol (Cold Spring Harb Protoc, 2010)
Equipment
Amicon Ultra-15 centrifugal filter unit, 3.5 kDa cutoff (Millipore Sigma, catalog number: UFC9030)
Anion-exchange column (HiTrapTM, Q FF, 5 mL)
Centrifuge (Kubota, Model-6500, serial number: K60115-G000)
Centrifuge for 50 and 1 mL tubes (Eppendorf, model: 5804R)
Dry bath (Bionova, model: SLM-DB-120)
Electrophoresis equipment (BIOBEE® Tech)
Electrophoresis power supply (Bio-Rad, PowerPacTM Basic, 041BR178399)
Fast protein liquid chromatography (FPLC), with fraction collector (GE Healthcare, ÄKTA prime plus)
Laminar airflow (local make)
Mastercycler (Eppendorf Flexi lid Nexus, 6333, serial number: 6333DQ408627)
Mixer (Eppendorf Mixmate 22331 Hamburg, serial number: 5353DN316975)
NanoDrop (Thermo Scientific, NanoDrop 2000c spectrophotometer)
Ni2+-NTA column (GE Healthcare HisTrapTM HP, 5 mL)
Peristaltic pump (Bio-Rad, Econo gradient pump, serial number: 491BR 1897, catalog number: 731-9002)
pH meter (Thermo ScientificTM Orion StarTM A111)
Rotor (Kubota, 6 × 50 mL- AG-506R)
Rotor (Kubota, 6 × 500 mL- AG-5006A)
S200 column (HiLoadTM 16/600 superdexTM 200pg, ID-0059)
SDS-PAGE setup (Invitrogen, Minigel tank)
Shaker and incubator (BioTek, NB-205VQ)
Sonicator (LABMAN Scientific Instruments, model: pro650)
Spectrophotometer (Eppendorf Biophotometer D30, serial number: 6133D0400689)
Tabletop centrifuge (Eppendorf centrifuge 5418, FA-45-18-11 S/N:20881)
Vacuum pump (Tarsons, Rockyvac 400)
Water filtration source (Millipore, Sigma)
Weighing balance, milligram sensitivity (Sartorius, BSA 623S-CW)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Manikandan, P., Nadig, K. and Singh, M. (2023). Large-scale Purification of Type III Toxin-antitoxin Ribonucleoprotein Complex and its Components from Escherichia coli for Biophysical Studies. Bio-protocol 13(13): e4763. DOI: 10.21769/BioProtoc.4763.
分类
微生物学 > 微生物生物化学 > 蛋白质 > 相互作用
生物化学 > RNA > RNA-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












