- EN - English
- CN - 中文
An ex vivo Model of Paired Cultured Hippocampal Neurons for Bi-directionally Studying Synaptic Transmission and Plasticity
成对培养的海马神经元的离体模型,用于双向研究突触传递和可塑性
发布: 2023年07月20日第13卷第14期 DOI: 10.21769/BioProtoc.4761 浏览次数: 2200
评审: Verena BurtscherMohammed Mostafizur RahmanWilly R Carrasquel-Ursulaez
Abstract
Synapses provide the main route of signal transduction within neuronal networks. Many factors regulate critical synaptic functions. These include presynaptic calcium channels, triggering neurotransmitter release, and postsynaptic ionotropic receptors, mediating excitatory and inhibitory postsynaptic potentials. The key features of synaptic transmission and plasticity can be studied in primary cultured hippocampal neurons. Here, we describe a protocol for the preparation and electrophysiological analysis of paired hippocampal neurons. This model system allows the selective genetic manipulation of one neuron in a simple neuronal network formed by only two hippocampal neurons. Bi-directionally analyzing synaptic transmission and short-term synaptic plasticity allows the analysis of both pre- and postsynaptic effects on synaptic transmission. For example, with one single paired network synaptic responses induced by both, a wild-type neuron and a genetically modified neuron can be directly compared. Ultimately, this protocol allows experimental modulation and hence investigation of synaptic mechanisms and thereby improves previously developed methods of studying synaptic transmission and plasticity in ex vivo cultured neurons.
Key features
• Preparation of ex vivo paired cultured hippocampal neurons.
• Bi-directional electrophysiological recordings of synaptic transmission and plasticity.
• Genetic modulation of synaptic network formation (demonstrated by presynaptic viral overexpression of the auxiliary calcium channel α2δ-2 subunit).
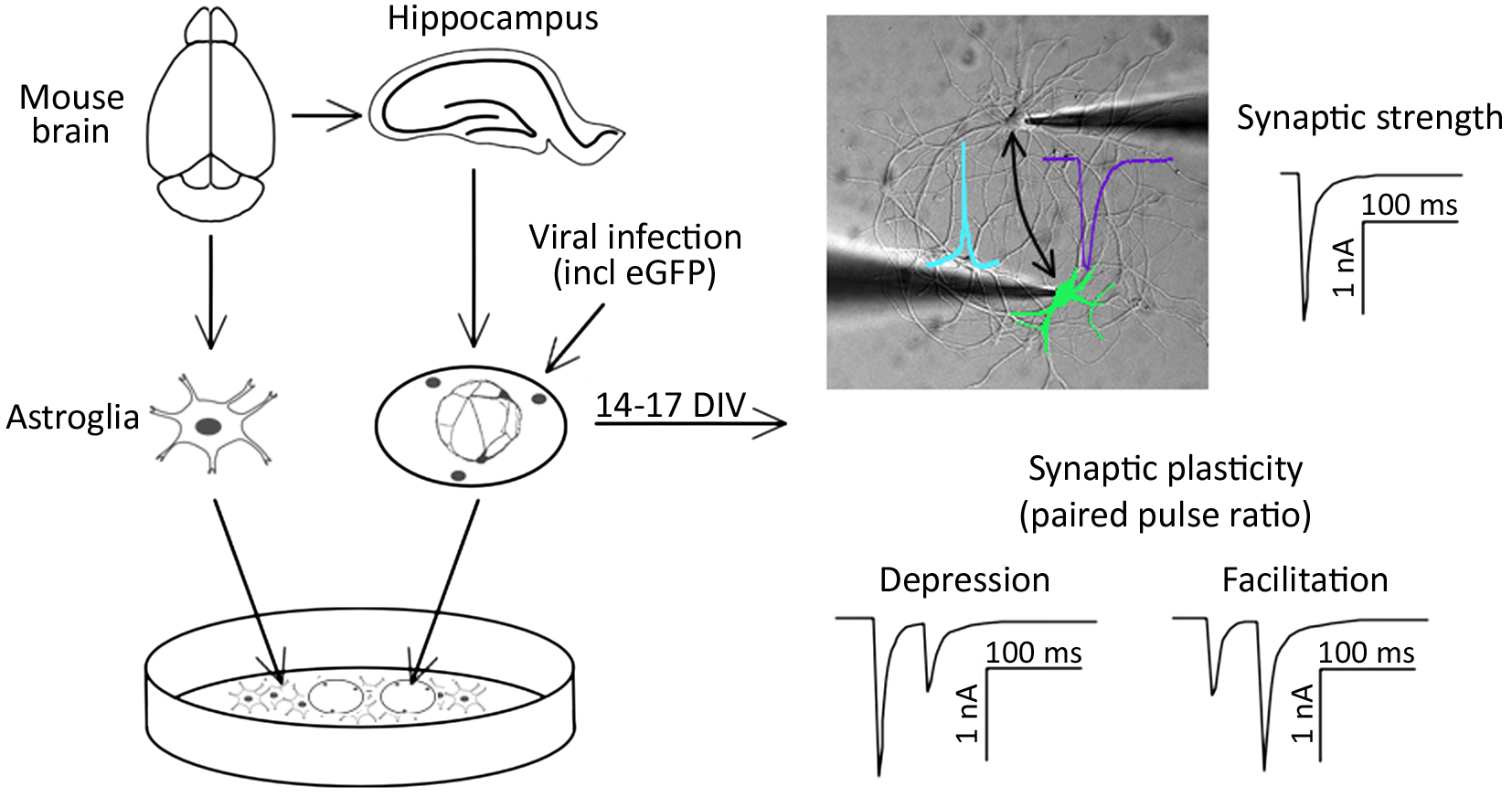
Graphical overview
Background
Signal transmission between neurons occurs via neurotransmitter release into the synaptic cleft. The temporal and spatial relation of pre- and post-synaptic firing modulates the strength of synaptic connections between neurons (Deperrois and Graupner, 2020). Two types of synaptic activity can be registered at the single cell level. First, miniature excitatory or inhibitory postsynaptic currents (mEPSC or mIPSC, respectively) appear as result of spontaneous local fusion of single synaptic vesicles. Second, excitatory and inhibitory postsynaptic currents (EPSC or IPSC, respectively) can be recorded in response to action potential firing by presynaptic glutamatergic or GABAergic neurons, respectively. Analysis of miniature postsynaptic potentials provides information on the amount and density of synaptic connections (frequency), as well as the postsynaptic receptor abundance (amplitude), and hence helps to study elementary synapse properties. However, higher levels of synaptic function, including the responses of synapses in regard to action potential firing, as well as short- and long-term adaptations of synaptic strength, require the analysis of evoked synaptic transmission. For example, paired-pulse stimulation protocols can serve as a basic model for studying short-term synaptic plasticity ex vivo (Bouteiller et al., 2010). Evoked synaptic transmission and plasticity in specific neuronal pathways can typically be studied in brain slices (Wang and Baudry, 2019). Alternatively, synaptic transmission and plasticity can be studied in cultured neurons, such as by employing optogenetic activation of neuronal cell populations (Barral and Reyes, 2017). In an acute brain slice, which is the standard model for the electrophysiological analysis of synaptic functions, presynaptic stimulation and postsynaptic responses can only be analyzed in one direction. However, as synaptic plasticity involves the possibility of changes in both pre- and post-synaptic components, one-directional measurements limit the study of mechanisms involved in modulating plasticity.
Here, we describe a protocol for culturing simple networks of paired hippocampal neurons for the bi-directional electrophysiological analysis of synaptic functions. This cellular ex vivo model has the following advantages: first, it allows the easy identification of the innervated cells. In classical neuronal cell cultures employing dispersed neurons, this is inherently difficult due to excessive branching of the axons and the possibility of hetero-synaptic innervation of the target neuron. Second, due to the defined simple network, all synapses are formed between the paired neurons. This results in increased amplitudes of postsynaptic responses and hence allows the reliable detection of changes in postsynaptic receptor function. Third, both cells of the cultured paired network can function as presynaptic (stimulated) and postsynaptic (innervated) neurons. Hence, this method allows recording synaptic transmission bi-directionally. This is particularly relevant in the context of genetic manipulations of one of the two paired neurons: as one of the paired neurons can be genetically modified by overexpression or knockdown of specific proteins, bi-directional stimulation protocols allow analyzing pre- and post-synaptic consequences in comparison with wild-type synaptic connections in the same neuronal network. As a proof of principle, we altered the expression of α2δ proteins, which act, on the one hand, as auxiliary subunits of voltage-gated calcium channels (Geisler et al., 2015; Ablinger et al., 2020; Dolphin and Obermair, 2022), and on the other hand as critical synaptic organizers (Eroglu et al., 2009; Geisler et al., 2019; Schöpf et al., 2021; Ablinger et al., 2022). Hence, we employed cultured paired hippocampal neurons to investigate the role of a splice variant of the α2δ-2 isoform in the trans-synaptic regulation of synapse formation and synaptic transmission, including short-term synaptic plasticity.
Materials and reagents
Animals
Timed pregnant wild-type mice (BALB/c, gestational age 16–17 days; Charles River Laboratories, Sulzfeld, Germany).
Biological materials
Lentiviral particles, carrying RNA encoding for the α2δ-2_ΔE23 splice variant and soluble eGFP as fluorescent marker (Geisler et al., 2019). Lentiviral particles were generated as previously described (Nasri et al., 2014; Benskey and Manfredsson, 2016).
Critical: Lentiviruses are classified as a biosafety level 2 (BSL-2) organism.
Materials
Surgical scissors, sharp blunt, straight 14.5 cm (Fine Science Tools, catalog number: 14001-14)
Tissue forceps, slim 1 × 2 teeth 10 cm (Fine Science Tools, catalog number: 11023-10)
Fine scissors, sharp, curved 10.5 cm (Fine Science Tools, catalog number: 14061-10)
Fine scissors, sharp, straight 10.5 cm (Fine Science Tools, catalog number: 14060-10)
Dumont #5 standard forceps (Fine Science Tools, catalog number: 11251-30)
Dumont #5 biology forceps (Fine Science Tools, catalog number: 11252-30)
Vannas-Tübingen spring scissors (Fine Science Tools, catalog number: 15004-08)
18 mm glass coverslips (Marienfeld Superior, catalog number: 0111580)
Rack for coverslips (custom build, Institute of Physiology, Medical University Innsbruck, Austria)
PTFE dish (Carl Roth, catalog number: K837.1)
12.5 cm filter paper (Carl Roth, catalog number: AP86.1)
Hemacytometer (Neubauer, catalog number: Brand 717805)
72 μm nylon mesh (Falcon, catalog number: 352350)
T75 flask (Falcon, catalog number: 353810)
Transfer pipette 3.5 mL (Sarstedt, catalog number: 86.1171.001)
15 mL centrifuge tube (Falcon, catalog number: 352070)
50 mL centrifuge tube (Falcon, catalog number: 352096)
60 mm plastic Petri dish (Falcon, catalog number: 353802)
60 mm Primaria plastic Petri dish (Falcon, catalog number: 353004)
15 cm glass Petri dish (Duran, catalog number: 237555201)
Pasteur pipette (Assistent, catalog number: 40567002)
5 mL serological pipette (Sarstedt, catalog number: 86.1253.001)
10 mL serological pipette (Sarstedt, catalog number: 86.1254.001)
25 mL serological pipette (Sarstedt, catalog number: 86.1685.001)
1.5 mL miniature spray (Rene Lezard)
Borosilicate glass with filament (Sutter Instrument, model: BF150-75-10)
2.5% trypsin (10×) (Gibco, catalog number: 15090-046)
0.5% Trypsin-EDTA (10×) (Gibco, catalog number: 15400-054)
B-27 supplement (50×) (Gibco, catalog number: 17504-044)
GlutaMAX (Gibco, catalog number: 35050-038)
Horse serum (Gibco, catalog number: 16050-122)
PenStrep (Penicillin-Streptomycin) (Gibco, catalog number: 15140-122)
MEM (Gibco, catalog number: 41090-028)
Neurobasal medium (Gibco, catalog number: 21103-049)
HBSS (10×) (Gibco, catalog number: 14180-046)
HEPES 1 M solution (Gibco, catalog number: 15630-056)
Poly-L-lysine (Sigma, catalog number: P2636)
Ara-C (Sigma, catalog number: C6645)
DNase (Sigma, catalog number: DN-25)
Sodium pyruvate (Sigma, catalog number: P2256)
Paraffin (Carl Roth, catalog number: X880.1)
Gelatine (Fluka, catalog number: 48722)
Nitric acid (Carl Roth, catalog number: 4989.2)
Glucose (Carl Roth, catalog number: HN06.3)
Boric acid (Sigma, catalog number: B6768)
Borax (sodium tetraborate decahydrate) (Sigma, catalog number: B9876)
Sodium chloride (NaCl) (Carl Roth, catalog number: 3957.1)
Potassium chloride (KCl) (Carl Roth, catalog number: 6781.3)
Calcium chloride dihydrate (CaCl2·2H2O) (Carl Roth, catalog number: 5239.2)
Magnesium chloride hexahydrate (MgCl2·6H2O) (Sigma, catalog number: M0250)
Sodium hydroxide (NaOH) (Carl Roth, catalog number: 6771.3)
Potassium hydroxide (KOH) (Carl Roth, catalog number: 6751.1)
Gluconic acid, potassium salt (K-gluconate) (Carl Roth, catalog number: 4621.1)
HEPES (Carl Roth, catalog number: 6763.1)
EGTA (Sigma, catalog number: E3889)
ATP, magnesium salt (Sigma, catalog number: A9187)
GTP, sodium salt (Sigma, catalog number: G8877)
Solutions
Pyruvate solution, 100 mM, 50 mL (see Recipes)
1% gelatine solution, 50 mL (see Recipes)
HBSS, 500 mL (see Recipes)
Glia medium, 500 mL (see Recipes)
Neuronal maintenance medium, 200 mL (see Recipes)
Neuronal plating medium, 200 mL (see Recipes)
1% DNase solution, 100 mL (see Recipes)
Sodium borate buffer, 500 mL (see Recipes)
Poly-L-lysine solution, 1 mg/mL (see Recipes)
EGTA solution, 0.5 M, 1 mL (see Recipes)
Extracellular solution, 100 mL, adjust pH 7.4 with NaOH (see Recipes)
Intracellular solution, 20 mL, adjust to pH 7.2 with KOH (see Recipes)
Sodium hydroxide solution, 1 M, 5 mL (see Recipes)
Potassium hydroxide solution, 1 M, 5 mL (see Recipes)
20% glucose solution, 50 mL (see Recipes)
Recipes
Pyruvate solution, 100 mM, 50 mL
Reagent Amount Sodium pyruvate 550 mg Milli-Q water Add to the total volume of 50 mL 1% gelatine solution, 50 mL
Reagent Amount Gelatine 500 mg Milli-Q water Add to the total volume of 50 mL HBSS, 500 mL
Reagent Amount HBSS (10×) 50 mL PenStrep 5 mL HEPES, 1 M 5 mL Milli-Q water 440 mL Glia medium, 500 mL
Reagent Amount MEM 430 mL PenStrep 5 mL Glucose, 20% 15 mL Horse serum 50 mL Neuronal maintenance medium, 200 mL
Reagent Amount Neurobasal medium 194 mL B-27 supplement, (50×) 4 mL GlutaMAX 2 mL Neuronal plating medium, 200 mL
Reagent Amount MEM 172 mL Pyruvate solution, 100 mM 2 mL Glucose, 20% 6 mL Horse serum 20 mL 1% DNase solution, 100 mL
Reagent Amount DNase 1 g HBSS 100 mL Sodium borate buffer, 500 mL
Reagent Final concentration Amount Boric acid 50 mM 1.54 g Borax (sodium tetraborate) 12.5 mM 2.376 g Milli-Q water Add to the total volume of 500 mL Poly-L-lysine solution, 1 mg/mL
Reagent Amount Poly-L-lysine 100 mg Sodium borate buffer 100 mL EGTA solution, 0.5 M, 1 mL
Reagent Amount EGTA 190.2 mg 1 M KOH Add to the total volume of 1 mL Extracellular solution, 100 mL, adjust pH 7.4 with 1 M NaOH solution
Reagent Final concentration Amount NaCl 137 mM 800.62 mg KCl 3 mM 22.36 mg Glucose 10 mM 180.2 mg HEPES 10 mM 238.3 mg CaCl2·2H2O 1.8 mM 26.46 mg MgCl2·6H2O 2 mM 40.66 mg Intracellular solution, 20 mL, adjust to pH 7.2 with 1 M KOH solution
Reagent Final concentration Amount K-gluconate 125 mM 585.62 mg KCl 10 mM 14.9 mg HEPES 10 mM 47.66 mg MgCl2·6H2O 1 mM 4.06 mg EGTA solution, 0.5M 2 mM 80 μL ATP (Mg) 4 mM 40.57 mg GTP (Na) 0.3 mM 3.14 mg Sodium hydroxide solution, 1 M, 5 mL
Reagent Amount NaOH 200 mg Milli-Q water Add to the total volume of 5 mL Potassium hydroxide solution, 1 M, 5 mL
Reagent Amount KOH 280.6 mg Milli-Q water Add to the total volume of 5 mL 20% glucose solution, 50 mL
Reagent Amount Glucose 10 g Milli-Q water Add to the total volume of 50 mL
Equipment
Stereo dissection microscope (Olympus, model: SZX2-ILLTQ)
Class II biological safety cabinet (Thermo Scientific, model: HERAsafe KS12)
Suction system (Welch, model: 112037-08)
Safety Bunsen burner (INTEGRA Biosciences, model: Fireboy Plus 144000)
Pipette controller (INTEGRA Biosciences, model: PIPETTEBOY acu 2)
Magnetic stirrer with heating (Phoenix Instrument, model: RMS-10HS)
CO2 incubator (Thermo Scientific, model: HERAcell 240i)
Water baths (VWR, catalog number: 462-0558)
pH meter (inoLab, model: pH 7110)
Micropipette puller (Sutter Instrument, model: P-97)
Microforge (Narishige, model: MF-830)
Ultrapure water purification system (Merck Millipore, model: Milli-Q® EQ 7000)
Universal oven (Memmert, model: UF260)
Experimental setup
CleanBench lab table (TMC, model: TM-63-9012S)
Inverted fluorescent microscope (Olympus, model: IX-83, equipped with LUCPLFLN40XPH/0.6 objective, Lumencor LED lamp, eGFP filter)
Microscope dependent platforms (Sutter Instrument, model: MDM-83-2)
Micromanipulators (Sutter Instrument, model: MPC-325-2)
Two-channel patch clamp amplifier (HEKA Elektronik, model: EPC 10 USB Double)
Quick change chamber RC-41LP (Harvard Apparatus, catalog number: 64-0368)
Software
PatchMaster v2x90.5 (HEKA Elektronik)
FitMaster v2x90.5 (HEKA Elektronik)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Stanika, R. and Obermair, G. J. (2023). An ex vivo Model of Paired Cultured Hippocampal Neurons for Bi-directionally Studying Synaptic Transmission and Plasticity. Bio-protocol 13(14): e4761. DOI: 10.21769/BioProtoc.4761.
- Geisler, S., Schöpf, C. L., Stanika, R., Kalb, M., Campiglio, M., Repetto, D., Traxler, L., Missler, M. and Obermair, G. J. (2019). Presynaptic α2δ-2 Calcium Channel Subunits Regulate Postsynaptic GABA(A) Receptor Abundance and Axonal Wiring. J. Neurosci. 39(14): 2581-2605.
分类
神经科学 > 细胞机理 > 突触生理学
生物物理学 > 电生理 > 膜片钳技术
细胞生物学 > 细胞信号传导 > 突触传递
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link