- EN - English
- CN - 中文
In vivo Electroporation of Skeletal Muscle Fibers in Mice
小鼠骨骼肌纤维的体内电穿孔
发布: 2023年07月05日第13卷第13期 DOI: 10.21769/BioProtoc.4759 浏览次数: 1830
评审: Salma MerchantAnonymous reviewer(s)
Abstract
In vitro models are essential for investigating the molecular, biochemical, and cell-biological aspects of skeletal muscle. Still, models that utilize cell lines or embryonic cells do not fully recapitulate mature muscle fibers in vivo. Protein function is best studied in mature differentiated tissue, where biological context is maintained, but this is often difficult when reliable detection reagents, such as antibodies, are not commercially available. Exogenous expression of tagged proteins in vivo solves some of these problems, but this approach can be technically challenging because either a mouse must be engineered for each protein of interest or viral vectors are required for adequate levels of expression. While viral vectors can infect target cells following local administration, they carry the risk of genome integration that may interfere with downstream analyses. Plasmids are another accessible expression system, but they require ancillary means of cell penetration; electroporation is a simple physical method for this purpose that requires minimal training or specialized equipment. Here, we describe a method for in vivo plasmid expression in a foot muscle following electroporation.
Graphical overview
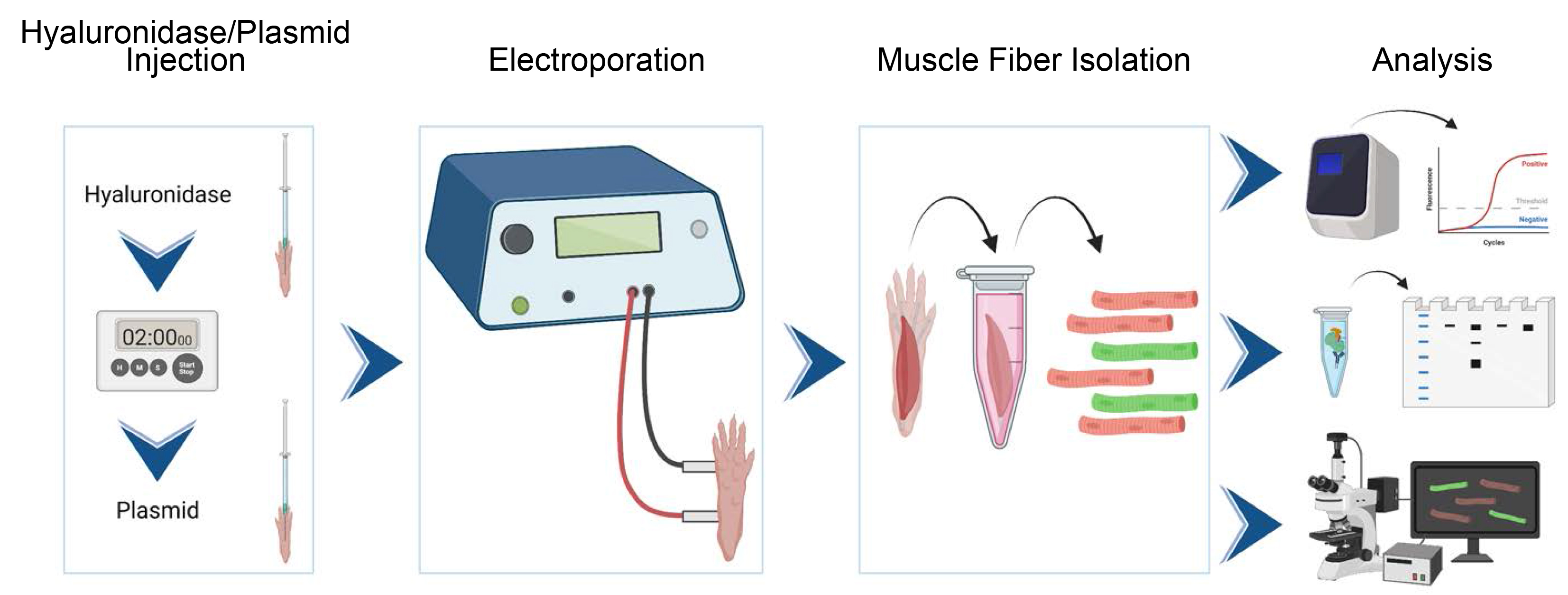
Background
Skeletal muscle is the largest human organ by mass and is essential for autonomous locomotion (Janssen et al., 2000). It is also the target of several degenerative genetic diseases collectively termed muscular dystrophies and myopathies. Recent improvements in genome sequencing have rapidly expanded the list of genes and gene variants causing muscle diseases, many of which have unknown or unconfirmed functions (Lek and MacArthur, 2014; Biancalana and Laporte, 2015; Angelini et al., 2018; Fichna et al., 2018). Besides the obvious desire to understand these novel disease-causing genes for therapeutic development, an exploration of their expression patterns and function, including cellular trafficking and localization, binding partners, and responses to various environmental stressors, is required to expand the current understanding of muscle cell biology. In vitro systems are convenient for the study of new gene products, as they consist of a single or defined mixture of cell types, are usually amenable to genetic manipulation (both knockout and overexpression), and deliver results quickly relative to in vivo systems. However, mature skeletal muscle fibers differ substantially from myotubes cultured in vitro in terms of size, structure, and three-dimensional organization (Dessauge et al., 2021). For example, nuclei regularly cluster in cultured myotubes but are separated into myonuclear domains in vivo. Furthermore, muscle in vivo consists primarily of contractile units (sarcomeres), which are interwoven with highly organized mitochondrial, t-tubule, and sarcoplasmic reticular systems, but typical muscle cell culture systems do not develop well-organized sarcomeres (Denes et al., 2019). These factors combine to influence expression, localization, and behavior of proteins in skeletal muscle fibers, which can differ substantively from what is observed in vitro (Deshmukh et al., 2015).
Historically, animal models (knockout or transgenic) have been the gold standard for probing protein function in vivo but are associated with substantial production costs and long characterization times. Another disadvantage of engineered mice is that a separate mouse is required for each protein of interest, and studying the behavior of several interacting proteins becomes daunting. One would like to use common in vitro tools, like fluorescent and biochemical tags, in vivo. Labeled proteins can be expressed via vectors in skeletal muscle, provided that the vector is able to enter the cell. Adeno-associated viral vectors can freely transduce muscle following intramuscular injection but are labor intensive to produce (Gregorevic et al., 2004; Qiao et al., 2011). Other viral vectors (e.g., lentivirus or retrovirus) can transduce stem cells in vitro, which can then be transplanted into muscle, but this is technically impractical in most cases (Li et al., 2005; Ousterout et al., 2015). A simpler approach is to transfect cells in vivo with plasmid DNA. This is efficiently accomplished through electroporation, a physical method for reversibly permeabilizing the cell membrane with strong electric field. Here, we describe a method for expressing tagged proteins in skeletal muscle fibers of the flexor digitorum brevis (FDB) muscle of the foot through direct intramuscular injection of plasmid DNA followed by electroporation. While this method is theoretically applicable to most skeletal muscles, the FDB possesses several advantages: it is small, accessible, and easily dissociable to generate a suspension of single muscle fibers for downstream analyses. This protocol provides a simple system for evaluating protein functions in skeletal muscle in vivo (Demonbreun and McNally, 2015; Foltz et al., 2021) with supplies that are attainable for most research labs.
Materials and reagents
Standard laboratory bulk materials
Microfuge tubes
Pipette tips
1 mL syringe
26 G needles
0.2 μm filters
Dissecting board and pins
Dissection scissors and forceps
Additional materials and reagents
Hyaluronidase (Sigma-Aldrich, catalog number: H4272); make a 0.5 mg/mL stock in sterile H2O, aliquot, and store at -20 °C
Isoflurane: must be stored in a locked drawer and accessible only by authorized users
Hank’s balanced salt solution (HBSS) (Thermo Fisher, Gibco, catalog number: 14025092)
Collagenase A (Roche, catalog number: 10103586001); prepare a 1% (w/v) stock solution in sterile DMEM, aliquot, and store for < 6 months at -20 °C
1 M HEPES (VWR, catalog number: 97064-360); store at room temperature
DMEM (Thermo Fisher, catalog number: 11995); store at 4 °C
Bovine serum albumin (BSA) (fraction V) (Roche, catalog number: 10775835001); store at 4 °C
CaCl2·2H2O (Sigma-Aldrich, catalog number: C5080); store at room temperature
MgSO4·7H2O (Sigma-Aldrich, catalog number: M5921); store at room temperature
KCl (Sigma-Aldrich, catalog number: P8041); store at room temperature
NaHCO3 (Fisher Scientific, catalog number: S233-500); store at room temperature
NaCl (Fisher Scientific, catalog number: BP358); store at room temperature
NaH2PO4·H2O (Sigma, catalog number: 9763); store at room temperature
100 mM sodium pyruvate (Thermo Fisher, catalog number: 11360070); store at 4 °C
D-Glucose (Sigma-Aldrich, catalog number: G7528); store at room temperature
MEM amino acids (Thermo Fisher, catalog number: 11130051); store at 4 °C
L-serine (Sigma, catalog number: S4311); store at room temperature
Glycine (Sigma, catalog number: G4392); store at room temperature
L-glutamine (Thermo Fisher, catalog number: 25030081); aliquot and store at -20 °C
Penicillin/streptomycin (Thermo Fisher, catalog number: 15140122); aliquot and store at -20 °C
Digestion buffer (see Recipes)
Imaging medium (optional) (see Recipes)
Equipment
Electroporator (BTX, model: ECM 830), Tweezertrodes platinum plated 7 mm (BTX, catalog number: 45-0488), or needle electrodes (2-needle array electrode 5 mm; BTX, catalog number: 45-0206)
Isoflurane flow regulator (Pro 5 oxygen concentrator 625), induction chamber, and nose cone
Cell culture incubator or bead bath at 37 °C (e.g., Fisher Scientific, model: Isotemp 210)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Foltz, S. J., Hartzell, H. C. and Choo, H. J. (2023). In vivo Electroporation of Skeletal Muscle Fibers in Mice. Bio-protocol 13(13): e4759. DOI: 10.21769/BioProtoc.4759.
- Foltz, S. J., Cui, Y. Y., Choo, H. J. and Hartzell, H. C. (2021). ANO5 ensures trafficking of annexins in wounded myofibers. J Cell Biol 220(3): e202007059.
分类
分子生物学 > DNA > 转染
发育生物学 > 细胞生长和命运决定 > 肌纤维
细胞生物学 > 组织分析 > 组织分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













