- EN - English
- CN - 中文
Human Schwann Cells in vitro I. Nerve Tissue Processing, Pre-degeneration, Isolation, and Culturing of Primary Cells
体外人雪旺细胞 I. 神经组织的加工、预变性、分离和原代细胞培养
发布: 2023年11月20日第13卷第22期 DOI: 10.21769/BioProtoc.4748 浏览次数: 3003
评审: Vivien J. Coulson-ThomasCarmen Melendez-VasquezHeleen van 't Spijker
Abstract
This paper presents versatile protocols to prepare primary human Schwann cell (hSC) cultures from mature peripheral nervous system tissues, including fascicles from long spinal nerves, nerve roots, and ganglia. This protocol starts with a description of nerve tissue procurement, handling, and dissection to obtain tissue sections suitable for hSC isolation and culturing. A description follows on how to disintegrate the nerve tissue by delayed enzymatic dissociation, plate the initial cell suspensions on a two-dimensional substrate, and culture the primary hSCs. Each section contains detailed procedures, technical notes, and background information to aid investigators in understanding and managing all steps. Some general recommendations are made to optimize the recovery, growth, and purity of the hSC cultures irrespective of the tissue source. These recommendations include: (1) pre-culturing epineurium- and perineurium-free nerve fascicles under conditions of adherence or suspension depending on the size of the explants to facilitate the release of proliferative, in vitro–activated hSCs; (2) plating the initial cell suspensions as individual droplets on a laminin-coated substrate to expedite cell adhesion and thereby increase the recovery of viable cells; and (3) culturing the fascicles (pre-degeneration step) and the cells derived therefrom in mitogen- and serum-supplemented medium to accelerate hSC dedifferentiation and promote mitogenesis before and after tissue dissociation, respectively. The hSC cultures obtained as suggested in this protocol are suitable for assorted basic and translational research applications. With the appropriate adaptations, donor-relevant hSC cultures can be prepared using fresh or postmortem tissue biospecimens of a wide range of types and sizes.
Keywords: Human Schwann cells (人施旺细胞)Background
Schwann cells (SCs) are a heterogeneous group of axon-ensheathing cells in the peripheral nervous system of all vertebrate species (Jessen et al., 2015). These nerve-resident neuroglial cells can be isolated and expanded in vitro using standard cell culture techniques. Human SC (hSC) cultures can be prepared using any type of nerve or ganglion from developing and adult organ donors [reviewed recently in Monje (2020)]. Culturing hSCs is a lengthier and more labor-intensive process than culturing SCs from rodents and other experimental animals. However, the hSCs obtained from an initial harvest can be amplified substantially in vitro to generate large numbers of cells for a wide range of experimental approaches (Figure 1). Once established, the hSC cultures can be managed in a manner similar to adherent cell lines, though there are limitations regarding the expandability of individual stocks.
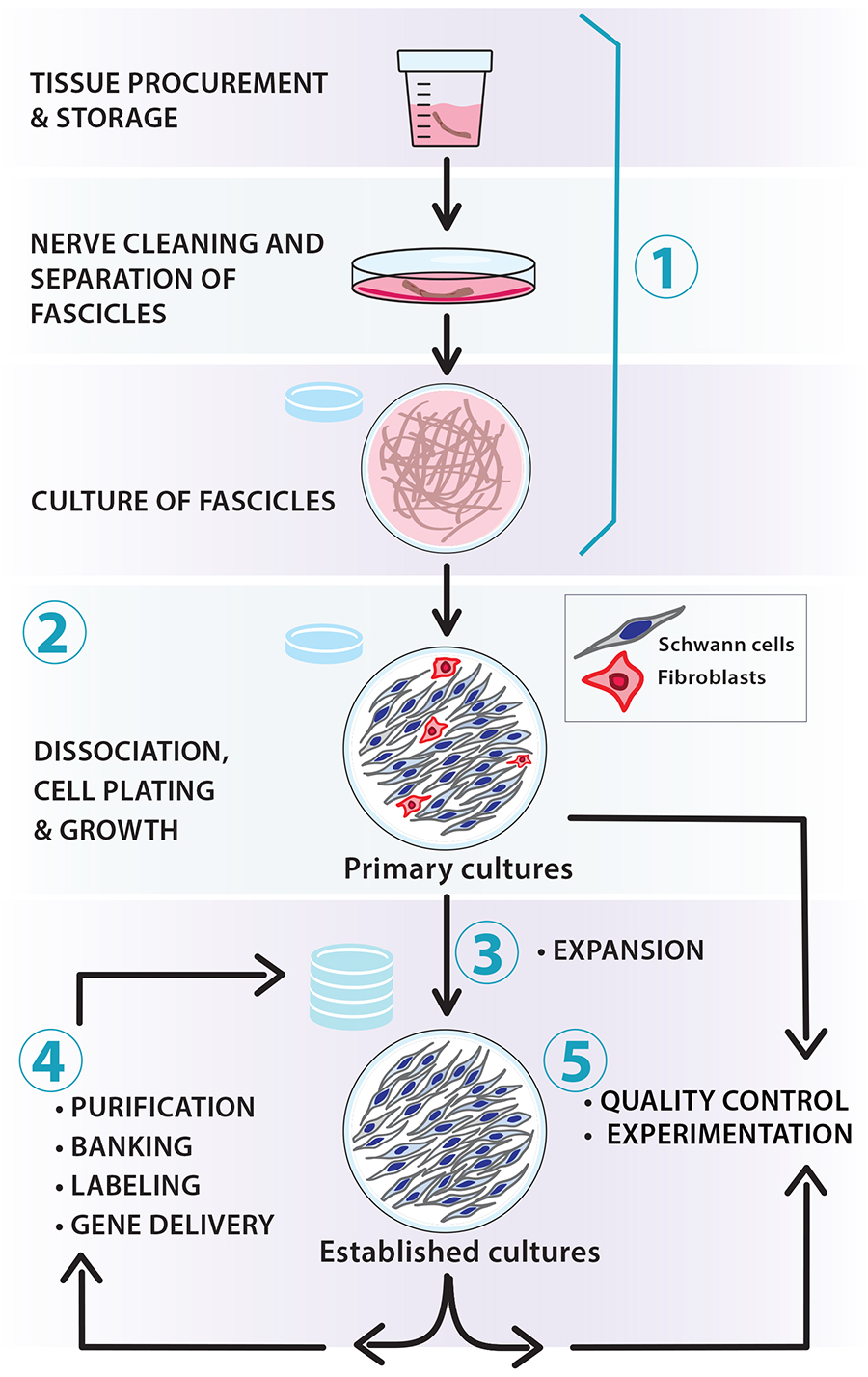
Figure 1. Scalable workflow for the preparation of nerve-derived human Schwann cell (hSC) cultures. The protocols described in this article and associated manuscripts (Monje, 2023a and 2023b) address the following basic procedures: (1) tissue procurement, dissection, and culture of fascicles (pre-degeneration); (2) enzymatic dissociation, isolation, and culture of primary hSCs; (3) derivation of established hSC cultures and amplification via serial passaging; (4) routine manipulations in vitro (e.g., purification, cryopreservation, labeling, and gene delivery); and (5) quality control of identity and bioactivity of the cells to be used in experimentation. Our protocols are adaptable; for instance, the number of hSCs can be scaled up by increasing the size of the tissue specimens used for cell isolation (primary cultures) and expanding the populations in medium containing mitogenic factors and serum (established cultures).
SC cultures from human tissues have facilitated numerous discoveries over nearly four decades [reviewed in Guest et al. (2013); Monje et al. (2021); Vallejo et al. (2022)]. These cultures are accurate in vitro models for studying neural development, differentiation, regeneration, electrophysiology, and toxicology in normal and disease states. They are also valuable for cell therapy development to repair damage caused by trauma and neurodegenerative disease. Indeed, the transplantation of cultured hSCs from a patient’s sural nerve has been implemented as a strategy to treat spinal cord and peripheral nerve injuries in USA-FDA-regulated clinical trials (Levi et al., 2016; Anderson et al., 2017; Khan et al., 2021).
Empirical data have shown that highly viable, expandable hSC cultures can be established from tissues provided by donors > 60 years of age [reviewed in Bunge et al. (2017)]. Biospecimens from live donors are not required. In fact, hSC cultures from postmortem tissues are phenotypically indistinguishable from those obtained from live donors (Boyer et al., 1994; Casella et al., 1996; Bastidas et al., 2017). Importantly, in vitro cultured SCs from adult nerves retain their ability to proliferate in response to axon contact, promote axonal growth, and form a myelin sheath (Morrissey et al., 1991), which are key functions of SCs during nerve development, maturation, and repair (Jessen et al., 2015).
Two main obstacles must be overcome to establish SC cultures from humans: (1) harvesting sufficient proliferative hSCs from the source tissue; and (2) expanding these primary hSCs sufficiently while maintaining low levels of fibroblast contamination (Morrissey et al., 1995; Peng et al., 2020). Processing adult biospecimens for cell isolation is more time demanding and challenging than doing so from developing (embryonic, neonatal) nerves. This is at least in part due to the presence of multiple layers of connective tissue (CT) and extracellular matrix (ECM), both within and around the SC-enriched fascicles. In addition, the highly elaborate cellular architecture of mature myelinating and ensheathing (non-myelinating or Remak) SCs, which are among the largest cells in the body, impose a hard-to-overcome technical barrier for disintegrating the tissue without disturbing the integrity of the cells. Although isolating hSCs immediately after nerve harvesting is feasible under certain conditions (Weiss et al., 2016), we strongly recommend pre-culturing the tissues without dissociation to achieve more consistent cell yields (Bunge et al., 2017; Chu et al., 2022). We also recommend selective immunological purification protocols to tackle the problem of fibroblast overgrowth, although other methods are available to enrich hSCs over non-glial cell populations.
In the following sections, we present generic protocols for preparing primary hSC cultures by including procedures for: (1) the dissection and harvesting of fascicles, roots, and ganglia from adult human donors; (2) the pre-degeneration of nerve segments, an intermediate step that involves the culturing of intact tissues to enrich the number of hSCs; (3) the disintegration of cultured tissues by proteolytic enzymes and the plating of initial cell suspensions on an adherent substrate; and (4) the growth and management of primary hSCs at the desired quality and quantity (Figures 1 and 2). Relevant notes on the procurement, storage, and handling of patient-derived biospecimens are included along with two different protocols for processing large and small tissue biospecimens. Various recommendations are made for managing the hSC cultures during the initial stages of growth. Supportive data on the properties of hSC cultures prepared according to these methods can be found in our published studies (Monje et al., 2006, 2008 and 2018). Our methodologies were developed using assorted tissue sources from deidentified donors. Importantly, the methods presented here are intended for non-clinical research only and differ substantially from those used in preclinical research (Bastidas et al., 2017) and clinical trials (Khan et al., 2021). However, it should be mentioned that in vitro cultured hSCs from nerves, skin, and ganglia are expected to exhibit fairly similar phenotypic and functional characteristics regardless of the tissue of origin and the mode of preparation (Stratton et al., 2017; Monje et al., 2018 and 2020; Chu et al., 2022).
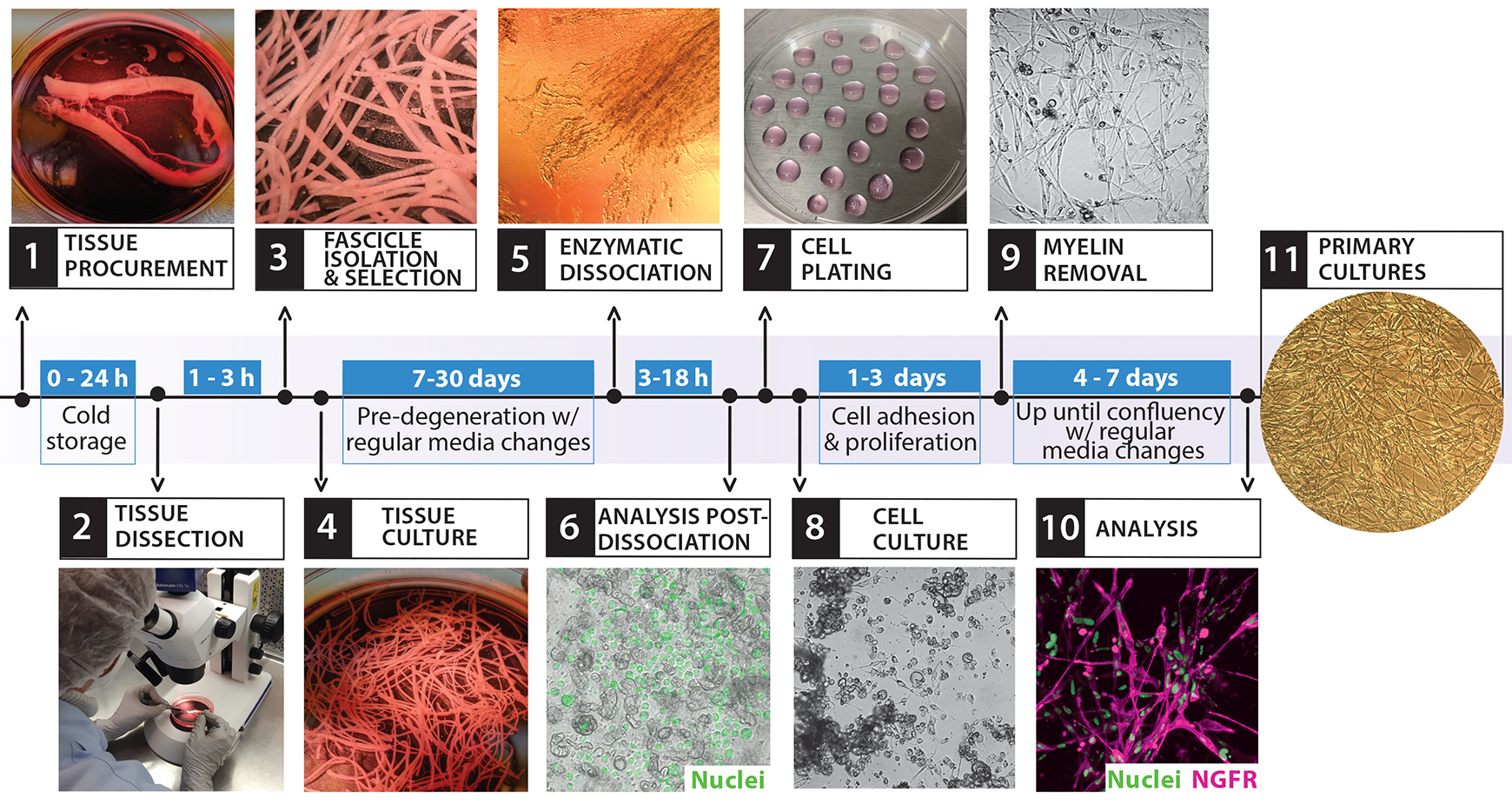
Figure 2. Isolation and culture of primary human Schwann cell (hSCs). The diagram depicts the overall cell culture strategy, highlighting the timeline for each procedure and the main steps involved, as illustrated by the representative images. This workflow starts with the bioprocessing of peripheral nerve tissues (steps 1–3) and ends with the establishment of confluent cultures of primary hSCs ready to use (step 11). The entire process can take at least three weeks, depending mostly on the length of the pre-degeneration phase (step 4, tissue culture).
Materials and reagents
All materials, reagents, and solutions should be endotoxin-free and cell culture grade. Except for dissection tools that are cleaned and sterilized for each procedure, most laboratory ware can be acquired as disposable materials for single use. It is recommended to use commercially available cell culture grade water to prepare all solutions, buffers, and culture media. In the sections below, we have added products’ information for reference only. We have mainly used disposable cell culture–treated flasks and dishes from CorningTM, but products from other brands may be equally suitable.
Supplies and consumables
Dumont forceps #3, #4, and #5; straight tip shape (Fine Science Tools, catalog numbers: 11231-30, 11242-30, and 11251-10, respectively)
Spring scissors, angled to side (Fine Science Tools, catalog number: 15006-09)
Moria Dowell spring scissors, straight tip shape (Fine Science Tools, catalog number: 15372-62)
Disposable 5 mL serological pipettes, polystyrene, sterile (VWR, catalog number: 89130-896)
Disposable 10 mL serological pipettes, polystyrene, sterile (VWR, catalog number: 19221005)
Polystyrene Pasteur pipettes, sterile and individually wrapped (VWR, Argos Technology, catalog number: 10122-560)
Borosilicate glass Pasteur (transfer) pipettes (Corning, catalog number: 7095D-9) with attached rubber bulb
100 mm Petri dishes (Corning, catalog number: 351029)
Polypropylene conical-bottom centrifuge tubes, 15 and 50 mL (Corning, catalog numbers: 430791 and 430290, respectively)
Round-bottom centrifuge tubes with a snap cap, polypropylene, 15 mL (BD, catalog number: 352059)
Polystyrene cell culture dishes, 35, 60, or 100 mm (Corning, catalog numbers: 353001, 353002, and 353003, respectively)
24-well plate, flat bottom (Corning, catalog number: 3524)
Containers with wet ice
Media, supplements, and reagents for cell culture
Distilled water, cell culture grade (Thermo Fisher Scientific, Gibco, catalog number: 15-230-147)
Dulbecco’s phosphate-buffered saline (DPBS), pH 7.2 (Thermo Fisher Scientific, Gibco, catalog number: 14190)
Hank’s balanced salt solution (HBSS), formulated without calcium or magnesium and containing phenol red, pH 7.2 (Thermo Fisher Scientific, Gibco, catalog number: 14170-112)
Leibovitz’s L15 medium (Thermo Fisher Scientific, Gibco, catalog number: 11415064)
Dulbecco’s modified Eagle’s medium (DMEM) with high glucose and phenol red, pH 7.2 (Thermo Fisher Scientific, Gibco, catalog number: 11965092)
1,000× Gentamycin (Thermo Fisher Scientific, Gibco, catalog number: 15750-060)
De-complemented, gamma-irradiated fetal bovine serum (FBS) (HyClone, catalog number: SV 30014.03)
100× GlutaMAX supplement (Thermo Fisher Scientific, Gibco, catalog number: 35050061)
Forskolin (Sigma-Aldrich, catalog number: F68861)
Heregulin-β1177-244 (referred to as heregulin) (Preprotech, catalog number: G-100-03)
Laminin stock solution, consisting of a sterile 1 mg/mL laminin from Engelbreth-Holm-Swarm murine sarcoma basement membrane (Sigma-Aldrich, catalog number: L2020). Store in aliquots at -80 °C and use as described in Andersen and Monje (2018)
Poly-L-lysine (PLL) stock solution (Sigma, catalog number: P-2636). Prepare, store in aliquots at -80 °C, and use as described in Andersen and Monje (2018)
Matrigel growth factor reduced basement membrane matrix, phenol red-free (BD Biosciences Discovery Labware, catalog number: 356231)
Dispase II or neutral protease, ≥ 0.8 units/mg protein, lyophilized powder (Roche, catalog number: 165-859)
Collagenase Type I, ≥ 125 units per milligram dry weight, dialyzed, lyophilized powder (Worthington, CLS-1, catalog number: 4196)
Dissection medium (DM) (see Recipes)
Low proliferation medium (LP) (see Recipes)
High proliferation medium (HP) (see Recipes)
Laminin coating solution (see Recipes)
10× enzymatic solution (see Recipes)
Antibodies and fluorescent dyes
Anti-NGFR mouse IgG monoclonal antibody, produced in-house from the HB-8737 hybridoma cell line (reactivity: human/primate-specific NGFR; American Type Culture Collection, ATCC). See Ravelo et al. (2018) for a step-by-step description of NGFR immunostaining in live and fixed hSC cultures. (Optional) Use the rabbit monoclonal antibody EP1039Y (Abcam, catalog number: ab52987).
Anti-O4 mouse IgM monoclonal antibody, produced in-house from the O4 hybridoma cell line (reactivity: human/rat/mouse/pig/other; kindly provided by Dr. Melitta Schachner). See Ravelo et al. (2018) for a step-by-step description of O4 immunostaining in live hSC cultures. (Optional) Use a commercially available purified O4 antibody (Novus Biologicals, catalog number: NL637)
Hoechst 33342 (Sigma, catalog number: B2261), prepared in water at 1 mg/mL
Syto-24 green, fluorescent nucleic acid stain (Invitrogen, catalog number: S75559)
Propidium iodide (PI) nucleic acid stain (Sigma, catalog number: P4170) prepared in water at 1 mg/mL. See Ravelo et al. (2018) for a step-by-step description of viability assays using PI and Hoechst 33342 or Syto-24 green
FluoroMyelin red (or green), fluorescent myelin stain (Invitrogen, catalog number: F34652)
FM 4-64FX, fixable analog of FM 4-64 membrane stain (Invitrogen, catalog number: F34653)
4’,6-Diamidino-2-Phenylindole, dilactate (DAPI) (Invitrogen, catalog number: D3571), prepared in water at 1 mg/mL
Equipment
Stereomicroscope with an attached digital camera (Zeiss, Stemi 305/Axio cam ER C52)
Double gooseneck fiber optics with intensity control for cool white light illumination (DolanJenner)
NIGHTSEA® Fluorescence Viewing System equipped with blue and green barrier filters (Electron Microscopy Sciences)
Inverted phase contrast microscope with an attached digital camera (VWR V5MP)
Inverted fluorescence microscope with an attached digital camera (Olympus IX71)
Benchtop centrifuge (Beckman Coulter, model: Allegra X-I2R)
Cell counter for automated counting of cells in suspension (Bio-Rad, TC20 Automated cell counter). (Optional) Hemocytometer for manual cell counting
CO2 cell incubator set up at 37 °C and 8%–9% CO2 (Thermo Fisher Scientific, Forma Steri-Cycle)
Biosafety cabinet, BL2 level (Thermo Fisher Scientific, 1300 Series A2)
Germinator 500, bench-top sterilizer (Cell Point, catalog number: 5517)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Aparicio, G. I. and Monje, P. V. (2023). Human Schwann Cells in vitro I. Nerve Tissue Processing, Pre-degeneration, Isolation, and Culturing of Primary Cells. Bio-protocol 13(22): e4748. DOI: 10.21769/BioProtoc.4748.
分类
神经科学 > 周围神经系统 > 施万细胞
细胞生物学 > 细胞分离和培养 > 细胞分离
细胞生物学 > 细胞分离和培养 > 单层培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link