- EN - English
- CN - 中文
Mass Spectrometry-based Lipidomics, Lipid Bioenergetics, and Web Tool for Lipid Profiling and Quantification in Human Cells
基于质谱的脂质组学、脂质生物能学和用于人类细胞中脂质分析和定量的网络工具
发布: 2023年08月20日第13卷第16期 DOI: 10.21769/BioProtoc.4742 浏览次数: 3008
评审: Alka MehraNeelanjan BoseJianhong ChingPrajita Pandey

相关实验方案

诱导型HIV-1库削减检测(HIVRRA):用于评估外周血单个核细胞中HIV-1潜伏库清除策略毒性与效力的快速敏感方法
Jade Jansen [...] Neeltje A. Kootstra
2025年07月20日 2460 阅读
Abstract
Lipids can play diverse roles in metabolism, signaling, transport across membranes, regulating body temperature, and inflammation. Some viruses have evolved to exploit lipids in human cells to promote viral entry, fusion, replication, assembly, and energy production through fatty acid beta-oxidation. Hence, studying the virus–lipid interactions provides an opportunity to understand the biological processes involved in the viral life cycle, which can facilitate the development of antivirals. Due to the diversity and complexity of lipids, the assessment of lipid utilization in infected host cells can be challenging. However, the development of mass spectrometry, bioenergetics profiling, and bioinformatics has significantly advanced our knowledge on the study of lipidomics. Herein, we describe the detailed methods for lipid extraction, mass spectrometry, and assessment of fatty acid oxidation on cellular bioenergetics, as well as the bioinformatics approaches for detailed lipid analysis and utilization in host cells. These methods were employed for the investigation of lipid alterations in TMEM41B- and VMP1-deficient cells, where we previously found global dysregulations of the lipidome in these cells. Furthermore, we developed a web app to plot clustermaps or heatmaps for mass spectrometry data that is open source and can be hosted locally or at https://kuanrongchan-lipid-metabolite-analysis-app-k4im47.streamlit.app/. This protocol provides an efficient step-by-step methodology to assess lipid composition and usage in host cells.
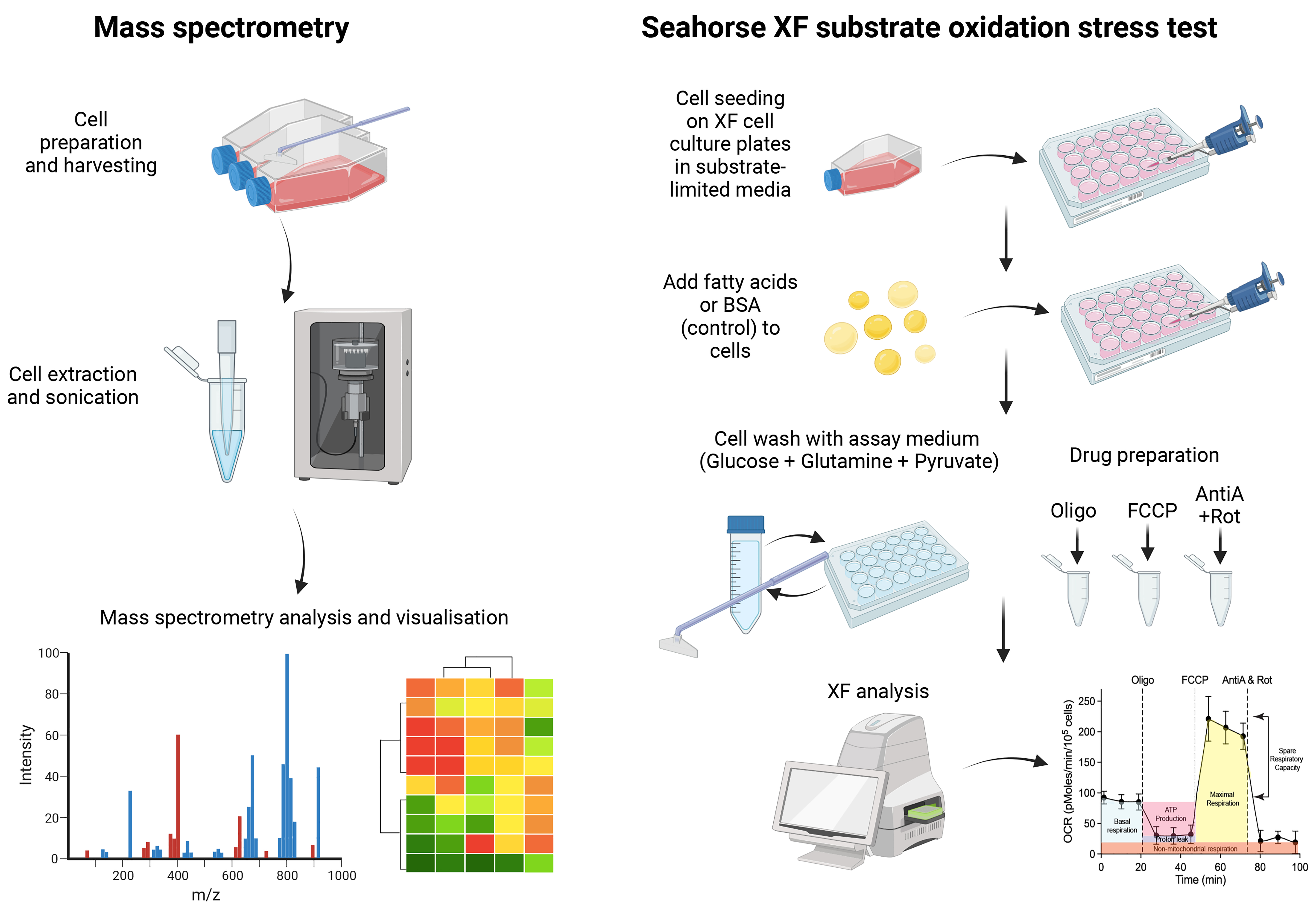
Graphical overview
Background
Lipids are critical for metabolism, signaling, transport across membranes, regulating body temperature, and inflammation. The dysregulation of lipid metabolism or lipid transport can thus disrupt the cell homeostasis and promote oxidative stress that leads to excessive inflammation and cell death. Interestingly, some viruses such as the enveloped viruses have evolved to hijack host cell lipids to augment viral fusion, formation of replication complexes, assembly, and egress. In addition, viruses can also modulate cellular lipid metabolic pathways and leverage intracellular lipid stores to promote viral replication. For instance, dengue virus (DENV) infection can upregulate and re-localize fatty acid synthase (FASN) to the replication complex to increase beta-oxidation for energy production (Heaton et al., 2010; Tang et al., 2014). DENV can also target lipid droplet stores to utilize triglycerides for the production of fatty acids, which are subsequently transported to the mitochondria where they undergo beta-oxidation to synthesize ATP required for viral replication (Zhang et al., 2017). However, much work remains to be done to better understand the virus and lipid interactions responsible for productive virus infection.
RNA interference and CRISPR technologies are useful tools to probe into the lipid metabolic processes required for viral infection. For example, we have recently identified that TMEM41B and VMP1 play a central role in lipid mobilization, mitochondrial beta-oxidation, and global metabolic regulations, to facilitate the replication of flaviviruses and coronaviruses (Yousefi et al., 2022). Deficiency of these proteins resulted in impaired beta-oxidation capacity and dysregulation of the cell lipidome that severely compromised viral infection. To gain insights into the lipid metabolism pathways that are differentially modulated before and after virus infection, we can leverage mass spectrometry to characterize the lipidome. The current protocol describes the detailed procedures required for mass spectrometry in target cells. We have used liquid chromatography–mass spectrometry (LC–MS), the preferred technique for use in metabolomics and lipidomics due to its ability to identify metabolites, even at low concentrations. The method also allows for untargeted lipidomics analysis, so users can simultaneously quantify a variety of lipids in host cells. Furthermore, to evaluate the effects of fatty acid oxidation on cellular bioenergetics, a modified Seahorse assay can be used to ascertain the lipid dependencies for energy production. In combination, these assays provide molecular insights into the role of lipid metabolism in cellular energetics and their potential contribution to virus infection outcome. Finally, we developed a web tool (https://kuanrongchan-lipid-metabolite-analysis-app-k4im47.streamlit.app/) that allows users to plot clustergrams and heatmaps based on the intensity measurements from the LC–MS. While we have used these methods to study virus–lipid interactions in human cells, as lipids are involved in multiple cell processes, we believe that these methods can be more broadly applied to study the role of lipids for other diseases, such as diabetes, obesity, atherosclerosis, and cancer.
In summary, we will describe the detailed protocols for:
Sample preparation and cell extraction for LC–MS
LC–MS analysis and interpretation
Bioinformatics analysis and web tool for data analysis
Seahorse bioenergetics analysis for fatty acid oxidation
Materials and reagents
HEK 293FT cells (Invitrogen, catalog number: R70007)
Dulbecco’s modified Eagle medium (DMEM) with L-glutamine and sodium pyruvate (Thermo Fisher Scientific, Gibco, catalog number: 11995)
0.22 μm filter (Merck Millipore, catalog number: SLGP0033RS)
Seahorse XF RPMI medium (without phenol red, bicarbonate, glucose, pyruvate, or glutamine, contains 1 mM HEPES, adjusted to pH 7.4) (Agilent, catalog number: 103576-100)
Seahorse XF calibrant solution [Agilent, 100 mL (catalog number: 03059-000) or 500 mL (catalog number: l100840-000)]
L-glutamine (200 mM) (Thermo Fisher Scientific, Gibco, catalog number: 25030081)
Fetal bovine serum (FBS) (HyClone, catalog number: SH30396.03)
Trypsin-EDTA (0.25%), phenol red (Thermo Fisher Scientific, Gibco, catalog number: 25200072)
10× PBS (Sigma-Aldrich, catalog number: BUF-2040-10X4L)
Additional materials required for mass spectrometry
Cell scraper (Thermo Fisher Scientific, catalog number: 08-100-241)
Tert-butyl methyl ether (MTBE), HPLC Plus (Sigma-Aldrich, catalog number: 650560-1L)
Methanol, hypergrade for LC-MS LiChrosolv® (Merck, Supelco, catalog number: 1.06035.2500)
Acetonitrile (ACN), hypergrade for LC-MS LiChrosolv® (Merck, Supelco, catalog number: 1.00029.2500)
Isopropanol, hypergrade for LC-MS LiChrosolv® (Merck, Supelco, catalog number: 1.02781.2500)
Ammonium formate (Honeywell Research Chemicals, Fluka, catalog number: 55674-50g-F)
Formic acid, LC/MS grade (Fisher Chemical, catalog number: A117-50)
Ultrapure water (H2O) (Sartorius Stedim Biotech, arium pro VF)
HPLC vial (Agilent, catalog number: 5182-0716)
Additional materials required for Seahorse assays
Seahorse XFe24 V7 PS cell culture microplate (Agilent, catalog number: 100777-004)
Linoleic acid (Sigma-Aldrich, catalog number: L9530-5ML)
Oleic acid (Sigma-Aldrich, catalog number: O3008-5ML)
Oligomycin A (Sigma-Aldrich, catalog number: 209-437-3)
Carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP) (Sigma-Aldrich, catalog number: 206-730-8)
Rotenone (Sigma-Aldrich, catalog number: 83-79-4)
Antimycin A from Streptomyces sp. (Sigma-Aldrich, catalog number: A8674)
Substrate-limited media (see Recipes)
Seahorse assay media (see Recipes)
10× drugs dilution (see Recipes)
Equipment
Seahorse XFe24 analyzer (Agilent, catalog number: 102238 or S7801A or S7801B)
Biological safety cabinet (Esco, BSC Class II)
5% CO2, 37 °C incubator
CO2-free 37 °C incubator
Sonicator (Elmasonic S 100 H, model: S100H)
Vorterxer (Thermo Scientific, model: M37610-33)
Centrifuge (Thermo Fisher Scientific, model: PICO 17)
TissueLyzer II (Qiagen)
Centrifuges for speed vacuum concentration (Labogene, model: Scan speed 40)
Cooling Trap for speed vacuum concentration (ScanLaf A/S, model: Coolsafe 110-4)
Mass spectrometry (Agilent Technologies iFunnel QTOF LC-MS, model: G6550B) (Note 2)
HPLC system (Agilent 1290 Infinity II, including High Speed Pump, Multisampler, Multicolumn Thermostat)
HPLC column, particle size of 1.8 μm, 2.1 mm × 100 mm (Agilent rapid resolution HD Zorbax SB-C18 column, catalog number: 858700-902)
Software
Seahorse Wave software (Agilent, https://www.agilent.com/en/product/cell-analysis/real-time-cell-metabolic-analysis/xf-software/seahorse-wave-desktop-software-740897)
Streamlit (Snowflake Inc., https://streamlit.io/)
Python (Python Software Foundation, https://www.python.org/)
MassHunter Qualitative Analysis 10.0 (Agilent Technologies)
MassHunter Profinder 10.0 (Agilent Technologies)
Mass Profiler Professional 15.1 (Agilent Technologies)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Cui, L., Yousefi, M., Yap, X., Koh, C. W. T., Tay, K. S. L., Ooi, Y. S. and Chan, K. R. (2023). Mass Spectrometry-based Lipidomics, Lipid Bioenergetics, and Web Tool for Lipid Profiling and Quantification in Human Cells. Bio-protocol 13(16): e4742. DOI: 10.21769/BioProtoc.4742.
分类
微生物学 > 微生物-宿主相互作用 > 病毒
细胞生物学 > 细胞新陈代谢 > 脂质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link