- EN - English
- CN - 中文
Catheterization of Pulmonary and Carotid Arteries for Concurrent Measurement of Mean Pulmonary and Systemic Arterial Pressure in Rat Models of Pulmonary Arterial Hypertension
肺动脉和颈动脉导管同时测量肺动脉高压大鼠模型的平均肺动脉压和系统动脉压
(*contributed equally to this work) 发布: 2023年08月20日第13卷第16期 DOI: 10.21769/BioProtoc.4737 浏览次数: 1783
评审: Ling LaiJordi Boix-i-CollAnonymous reviewer(s)
Abstract
Pulmonary hypertension (PH) is a group of pulmonary vascular disorders in which mean pulmonary arterial pressure (mPAP) becomes abnormally high because of various pathological conditions, including remodeling of the pulmonary arteries, lung and heart disorders, or congenital conditions. Various animal models, including mouse and rat models, have been used to recapitulate elevated mPAP observed in PH patients. However, the measurement and recording of mPAP and mean systemic arterial pressure (mSAP) in small animals require microsurgical procedures and a sophisticated data acquisition system. In this paper, we describe the surgical procedures for right heart catheterizations (RHC) to measure mPAP in rats. We also explain the catheterization of the carotid artery for simultaneous measurement of mPAP and mSAP using the PowerLab Data Acquisition system. We enumerate the surgical steps involved in exposing the jugular vein and the carotid artery for catheterizing these two blood vessels. We list the tools used for microsurgery in rats, describe the methods for preparing catheters, and illustrate the process for inserting the catheters in the pulmonary and carotid arteries. Finally, we delineate the steps involved in the calibration and setup of the PowerLab system for recording both mPAP and mSAP. This is the first protocol wherein we meticulously explain the surgical procedures for RHC in rats and the recording of mPAP and mSAP. We believe this protocol will be essential for PH research. Investigators with little training in animal handling can reproduce this microsurgical procedure for RHC in rats and measure mPAP and mSAP in rat models of PH. Further, this protocol is likely to help master RHC in rats that are performed for other conditions, such as heart failure, congenital heart disease, heart valve disorders, and heart transplantation.
Keywords: Pulmonary hypertension (肺动脉高压)Background
Pulmonary hypertension (PH) encompasses a group of pulmonary vascular disorders with varying etiologies. Depending on the cause and site of the pathogenesis, PH is classified into five groups and many subgroups (Sahay, 2019; Sarah et al., 2021). Despite the differences in the pathological basis of various groups of PH, the disease shares a common definition: a pathological condition in which the mean pulmonary arterial pressure (mPAP) is greater than 25 mmHg at rest or 30 mmHg during exercise (Galiè et al., 2016; Simonneau et al., 2019). Of the five groups of PH, pulmonary arterial hypertension (PAH) and its various subgroups fall in Group I (Wijeratne et al., 2018). In the case of PAH, pulmonary arteries and arterioles undergo pathological changes and thus become thicker and stiffer, resulting in elevated mPAP (Lai et al., 2014; Lan et al., 2018). In other forms of PH (Groups II–V), lung or heart diseases or other conditions increase mPAP (Galiè et al., 2016; Simonneau and Hoeper, 2019).
Because the chief manifestation of PH is increased mPAP, animal models for PH are developed to recapitulate elevated mPAP (Liu and Yan, 2022; Wu et al., 2022). Of the various animal models for PH, Sugen/hypoxia and monocrotaline-induced rat models of PH are two commonly used models (Stenmark et al., 2009; Vitali et al., 2014; Sztuka and Jasinska-Stroschein, 2017) (Figure 1). To develop hypoxia-based models, animals are first treated with a subcutaneous injection of 20 mg/kg Sugen 5416, a vascular endothelial growth factor receptor antagonist. Then, they are housed in 10% oxygen for 3–4 weeks (Bhat et al., 2017; Honda et al., 2020). A right heart catheterization (RHC) is performed to measure mPAP soon after the removal of the animals from the hypoxic environment or after three weeks of hypoxia followed by one week of normoxia (V. Gupta et al., 2013; Nahar et al., 2014; N. Gupta et al., 2015 and 2017; Nahar et al., 2016; Rashid et al., 2017, 2018a, 2018b and 2019; Keshavarz et al., 2019; Al-Hilal et al., 2021). In the case of monocrotaline-based models, animals receive a single subcutaneous injection of 30–80 mg/kg monocrotaline (Lachant et al., 2018; Jing et al., 2020), an alkaloid that causes PH by injuring pulmonary arterial endothelium (Xiao et al., 2017). The mPAP is measured 3–4 weeks after injection (Gomez-Arroyo et al., 2012; N. Gupta et al., 2017). In both models, mPAP rises to as high as 40–60 mmHg.
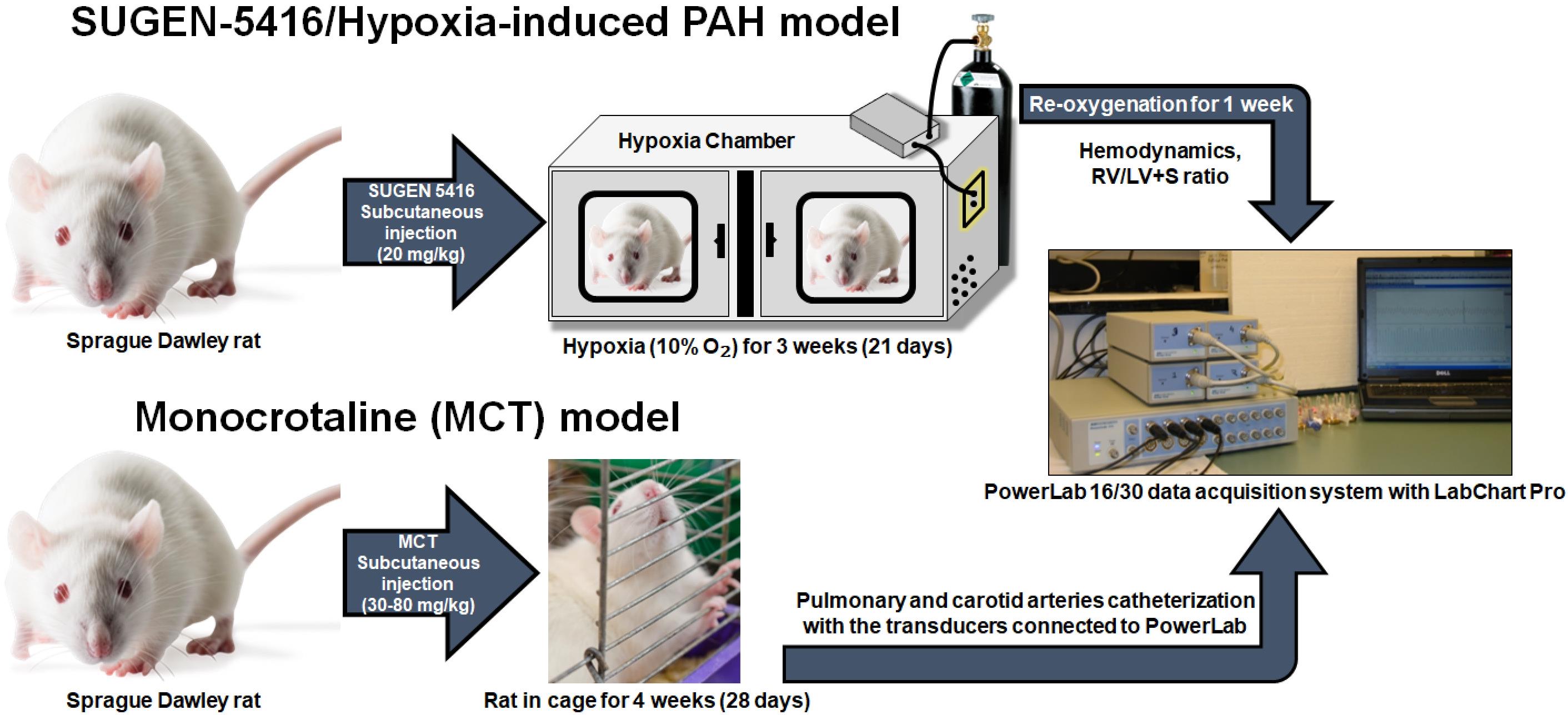
Figure 1. Sugen-5416/Hypoxia-induced pulmonary arterial hypertension (PAH) model and monocrotaline (MCT) model. For the development of Sugen/hypoxia-based models, animals are first treated with a subcutaneous injection of 20 mg/kg Sugen 5416 and then are housed in 10% oxygen for 3–4 weeks. A right heart catheterization (RHC) is performed to measure mean pulmonary arterial pressure (mPAP) soon after the removal of the animals from the hypoxic environment or after three weeks of hypoxia followed by one week of normoxia. In case of monocrotaline-based models, animals receive a single subcutaneous injection of 30–80 mg/kg monocrotaline. The mPAP is measured 3–4 weeks after MCT injection.
In fact, over the past several decades, these models have been used for studying PH pathophysiology and investigating the efficacy of drugs in reducing mPAP (V. Gupta et al., 2013; Maarman et al., 2013; Nahar et al., 2014; N. Gupta et al., 2015 and 2017; Nahar et al., 2016; Rashid et al., 2017, 2018a, 2018b and 2019; Keshavarz et al., 2019; Al-Hilal et al., 2021; Dignam et al., 2022). For mechanistic studies or to evaluate drugs’ efficacy in ameliorating pathological alterations, pulmonary arteries/arterioles are collected, and various histopathological studies are performed. Further, PH is treated with vasodilators to reduce elevated mPAP (Rose-Jones and McLaughlin, 2015). Reduced mPAP increases survival by reducing right ventricular hypertrophy, the chief cause of death in PH patients (Ryan et al., 2015; Zhu et al., 2019; Benza et al., 2022). However, vasodilator therapy also reduces blood pressure, resulting in peripheral hypotension–induced side effects (Packer, 1985; Rich, 2009; Humbert et al., 2014; Chida-Nagai et al., 2020). Thus, for studying the efficacy of investigational and commercially available drugs in animal models of PH, mPAP is measured as a functional endpoint for therapeutic outcomes (V. Gupta et al., 2013; Maarman et al., 2013; Nahar et al., 2014; N. Gupta et al., 2015 and 2017; Nahar et al., 2016; Rashid et al., 2017, 2018a, 2018b and 2019; Keshavarz et al., 2019; Suen et al., 2019; Al-Hilal et al., 2021; Beckmann et al., 2022; Dignam et al., 2022). Mean system arterial pressure (mSAP) is also measured to evaluate the influence of the drug on peripheral blood pressure or to assess whether the drug or formulation has pulmonary selectivity, by comparing the extent of reduction in mPAP and mSAP in the same rats. In a series of studies, we have shown that pulmonary selectivity can be evaluated by comparing mPAP and mSAP upon administration of various drug therapies to PH rats (V. Gupta et al., 2013; Maarman et al., 2013; Nahar et al., 2014; N. Gupta et al., 2015 and 2017; Nahar et al., 2016; Rashid et al., 2017, 2018a, 2018b and 2019; Keshavarz et al., 2019; Al-Hilal et al., 2021; Dignam et al., 2022).
To measure mPAP, an RHC is performed to place the catheter in the pulmonary artery. Likewise, for mSAP, a catheter is placed in the carotid artery of the same rats that underwent RHC. In our published studies, we inserted polyethylene (PE) catheters in both pulmonary and carotid arteries (N. Gupta et al., 2014; Nahar et al., 2014; Rashid et al., 2018b). However, insertion of PE catheter in pulmonary arteries is challenging because catheters require a specialized curvature for maneuvering from the jugular vein through the right atrium and right ventricle and finally to the pulmonary artery. Further, exposure to the jugular vein for RHC and carotid artery requires microsurgical procedures and specialized tools. Similarly, recording mPAP and mSAP entails using a specialized instrument. PowerLab, a system connected to bridge amplifiers and equipped with Lab Chart Pro software, is universally used to record mPAP and mSAP (Doggett et al., 2018). However, this system must be calibrated and set up accurately.
While we and others have been performing RHC to measure mPAP and mSAP using PowerLab systems for many years now (Rey et al., 2012; V. Gupta et al., 2013; Nahar et al., 2014; N. Gupta et al., 2015 and 2017; Nahar et al., 2016; Rashid et al., 2017, 2018a, 2018b and 2019; Keshavarz et al., 2019; Al-Hilal et al., 2021), there are no published protocols that list the surgical tools, explain the steps for preparation and insertion of catheters, and describe the setup and calibration of PowerLab lab data acquisition systems. In the absence of such a protocol, a new investigator may take months to establish the methodologies for RHC in rats and recording of mPAP and mSAP using the PowerLab system. Importantly, the unavailability of a thorough protocol is a major reason for significant delays in training new lab personnel in RHC or the transfer of methods from one investigator to another in the same lab. Thus, there is an important need for a detailed protocol for performing RHC in rats and measuring such; in this protocol paper, we put together all steps and processes involved in RHC and measurement of mPAP and mSAP, so that investigators with no training can perform the surgery and record mPAP and mSAP. This protocol can also be deployed in performing RHC in animal models for heart failure, congenital heart disease, heart valve disorders, and heart transplantation.
Materials and reagents
Air-TiteTM All-Plastic Henke-JectTM syringes (Fisher Scientific, catalog number: 14-817-25)
Stainless-steel flat instrument tray (Medicus Health, catalog number: 2841M1)
Mayo scissors, straight (Roboz, catalog number: RS-6872) (Figure 2A)
Surgical ophthalmic Westcott tenotomy scissors (Codman, catalog number: 28 54-6513) (Figure 2B)
Vannas spring scissors (Fine Science Tools, catalog number: 91500-09) (Figure 2C)
Vannas micro dissecting spring scissors (Roboz, catalog number: RS-5640) (Figure 2D)
Angled vessel cannulation forceps (Fine Science Tools, catalog number: 18403-11) (Figure 2E)
Serrated Semken forceps (Fine Science Tools, catalog number: 11008-13) (Figure 2F)
Extra fine Graefe forceps, quantity: 2 (Fine Science Tools, catalog number: 11150-10) (Figure 2G)
Graefe forceps, quantity: 2 (Roboz, catalog number: RS-5138) (Figure 2H)
Delicate suture tying forceps (Fine Science Tools, catalog number: 11063-07) (Figure 2I)
Micro serrefines (Fine Science Tools, catalog number: 18055-01) (Figure 2J)
Vein pick (Braintree Scientific Inc., catalog number: V-PIC) (Figure 2K)
25 G needle (Sigma Aldrich, catalog number: Z192406) (Figure 2L)
16 G needle (Becton Dickinson, catalog number: 305198)
HSW syringe 1 mL capacity, polypropylene (Grainger, catalog number: 45UC66)
Silk sutures, non-absorbable, 5-0 (Braintree Scientific, catalog number: SUT-S-106)
PE-50 catheter: polyethylene .023" × .038" per ft. (Braintree Scientific, catalog number: PE50)
Umbili-CathTM 3.5 French single lumen polyurethane umbilical vessel catheter (UVC) (Utah Medical Products, catalog number: 4183505)
Surgical stainless-steel suture (Ethicon, catalog number: DS24)
Acrylic sheet, 6 in. × 6 in., 0.25 inch thick (McMaster-Carr, catalog number: 8560K358)
Umbili-CathTM 3.5 French dual lumen silicone UVC, marked to 34 cm, 20/23 gauge (Utah Medical Products, catalog number: 4273505)
Safelet IV catheter 20 gauge 1" Luer tapered end Teflon (Henry Schein Medical, catalog number: 1198184)
High temperature cautery kit (Fine Science Tools, catalog number: 18010-00)
3MTM MicroporeTM surgical paper tape (Fisher Scientific, catalog number: 19-027761)
Betadine® microbicide solution (Fisher Scientific, catalog number: 19-027136)
Gauze pad (Fisher Scientific, catalog number: 22-362178)
Sprague Dawley rats (Charles River Laboratory, stock number: 400SASSD)
Deionized water (Barnstead Mega-Pure D2, Thermo Scientific)
Sodium chloride, 0.9% (w/v), isotonic saline, Ricca Chemical (Fisher Scientific, catalog number: 7647-14-5)
99% isopropyl alcohol, IPA (VWR, catalog number: IX0235)
Isoflurane liquid (Pharmacompass, NDC: 66794-017-10)
Ketamine hydrochloride, 100 mg/mL (Covetrus, NDC: 11695-0703-1)
Xylazine 20 mg/mL (Heartland Veterinary Supply and Pharmacy, catalog number: 343720-RX, NDC: 59399-110-20)
The cannulation tools shown in Figure 2 are listed in detail in the Materials and reagents section.
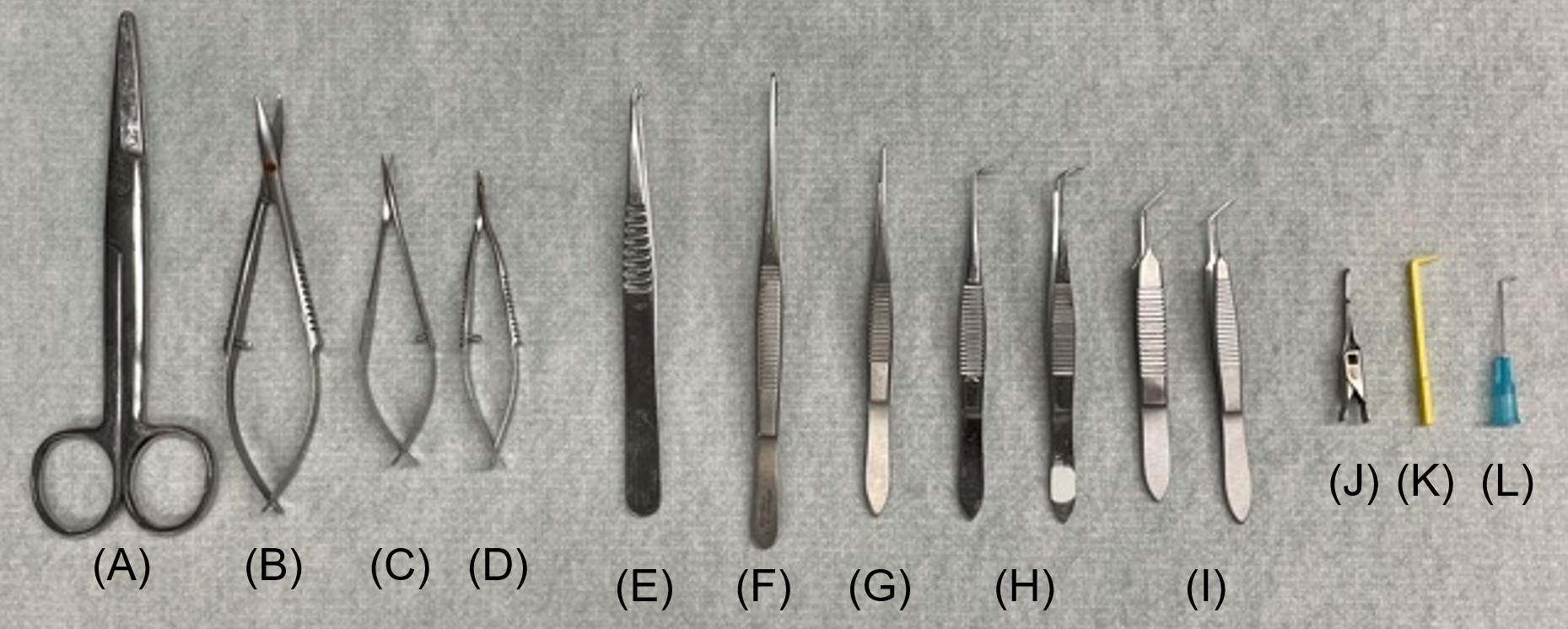
Figure 2. Canulation tools. (A) Mayo scissors; straight, (B) surgical ophthalmic Westcott tenotomy scissors, (C) Vannas spring scissors, (D) Vannas micro dissecting spring scissors, (E) angled vessel cannulation forceps, (F) serrated Semken forceps, (G) extra fine Graefe forceps, (H) Graefe forceps, quantity: 2, (I) delicate suture tying forceps, quantity: 2, (J) micro serrefines, (K) vein pick, (L) 25 G needle.
Equipment
Windows desktop computer
PowerLab ML880 16 Channel (ADInstruments)
Bridge Amplifier ML221 (ADInstruments)
Anesthesia induction chamber (Harvard Apparatus, catalog number:75-2030)
Wahl® Trimmer Combo kit (Kent Scientific, catalog number: CL9990-KIT)
SP844 medical pressure transducer sensor (Memscap)
Blood pressure transducer cable kit MLT1199 (Harvard Apparatus, catalog number: 77-0124)
Disposable clip-on BP domes (AD Instruments, catalog number: MLA844)
Blue 3-way stopcock, 2 female Luer locks, swivel male Luer lock (Qosina, catalog number: 99740)
3.5×–90× trinocular stereo zoom inverted light microscope (Amscope, catalog number: SM-3TZ-54S-5M)
Red 3-way stopcock, 2 female Luer locks, swivel male Luer lock (Qosina, catalog number: 99761)
Delta-Cal pressure transducer (Utah Medical Products Inc., catalog number: 650-950)
M3000 tabletop isoflurane, non-rebreathing anesthesia machine (Supera Anesthesia Innovations)
Heated small animal operating table (Harvard Apparatus, catalog number: 50-1247)
Software
Lab Chart Pro Version 7.3.8 (ADInstruments)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Sarkar, T., Isbatan, A., Moinuddin, S. M., Chen, J. and Ahsan, F. (2023). Catheterization of Pulmonary and Carotid Arteries for Concurrent Measurement of Mean Pulmonary and Systemic Arterial Pressure in Rat Models of Pulmonary Arterial Hypertension. Bio-protocol 13(16): e4737. DOI: 10.21769/BioProtoc.4737.
分类
医学 > 心血管疾病 > 心脏组织培养技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











