- EN - English
- CN - 中文
Heterologous Production of Artemisinin in Physcomitrium patens by Direct in vivo Assembly of Multiple DNA Fragments
通过在体内直接组装多个DNA片段在青蒿中异源生产青蒿素
发布: 2023年07月20日第13卷第14期 DOI: 10.21769/BioProtoc.4719 浏览次数: 2333
评审: Alba BlesaNazrin Abd AzizKrishna SaharanAnonymous reviewer(s)

相关实验方案

放射性示踪剂喂养实验测定紫草(聚合草)叶的吡咯里西啶生物碱生物合成能力及其合成产物的分析
Thomas Stegemann [...] Dietrich Ober
2018年02月05日 7285 阅读
Abstract
The sesquiterpene lactone compound artemisinin is a natural medicinal product of commercial importance. This Artemisia annua–derived secondary metabolite is well known for its antimalarial activity and has been studied in several other biological assays. However, the major shortcoming in its production and commercialization is its low accumulation in the native plant. Moreover, the chemical synthesis of artemisinin is difficult and expensive due to its complex structure. Hence, an alternative and sustainable production system of artemisinin in a heterologous host is required. Previously, heterologous production of artemisinin was achieved by Agrobacterium-mediated transformation. However, this requires extensive bioengineering of modified Nicotiana plants. Recently, a technique involving direct in vivo assembly of multiple DNA fragments in the moss, P. patens, has been successfully established. We utilized this technique to engineer artemisinin biosynthetic pathway genes into the moss, and artemisinin was obtained without further modifications with high initial production. Here, we provide protocols for establishing moss culture accumulating artemisinin, including culture preparation, transformation method, and compound detection via HS-SPME, UPLC-MRM-MS, and LC-QTOF-MS. The bioengineering of moss opens up a more sustainable, cost effective, and scalable platform not only in artemisinin production but also other high-value specialized metabolites in the future.
Keywords: Physcomitrium patens (小立碗藓)Background
Artemisinin is a secondary metabolite produced by the plant Artemisia annua. It is a sesquiterpene lactone with a unique endoperoxide 1,2,4-trioxane ring. This natural compound has been demonstrated to possess a range of biological activities, of which the most known is as an antimalarial drug. However, its low accumulation in the native plant resulted in a high market price, which becomes a major hindrance in its commercial production. Chemical synthesis is difficult due to its complex chemical structure, hence being not economically feasible. Efforts have been made toward the utilization of microbial production systems for increased artemisinin yield, which resulted in a partial synthesis of the compound requiring additional chemical steps (Ro et al., 2006; Paddon and Keasling, 2014). Plant heterologous hosts such as tobacco have been explored, requiring extensive bioengineering (Ting et al., 2013; Malhotra et al., 2016; Wang et al., 2016), yet producing limited artemisinin (Ikram et al., 2015).
Physcomitrium patens is a plant-based production system with important applications in biotechnology research (Simonsen et al., 2009; Ikram et al., 2015). It is a non-vascular plant that has low metabolic and chemical diversity compared to the higher plants (Bach et al., 2014), due to the low number of cytochromes P450 and UDP glycosyltransferases present in its genome (Yonekura-Sakakibara and Hanada, 2011; Hamberger and Bak, 2013). This attribute confers the advantage of reduced risks in unspecific modification by endogenous enzymes and products via pathways in higher plants for the detoxification of xenobiotics (Bach et al., 2014). P. patens is characterized by a simple terpenoid profile with a genome that possesses only a single diterpene synthase (Chen et al., 2011). Its genome is fully sequenced, has a haploid life cycle, and has an efficient homologous recombination machinery similar to yeast, making it an attractive industrial production system compared to other plant heterologous hosts (Lang et al., 2018; Decker and Reski, 2020). In addition, King et al. (2016) developed an orthodox transformation technology that involved the in vivo arrangement of DNA fragments in P. patens, further giving credence to its application as a photosynthetic chassis for heterologous natural products.
To produce artemisinin in P. patens, five genes—namely amorpha-4,11-diene synthase (ADS), cytochrome P450 (CYP71AV1), alcohol dehydrogenase 1 (ADH1), double-bond reductase 2 (DBR2), and aldehyde dehydrogenase 1 (ALDH1)—were introduced into the moss protoplast, being responsible for the biosynthesis of dihydroartemisinic acid that is subsequently converted into artemisinin by photooxidation (Figure 1). The five genes, consisting of three transformation sets, were transformed into P. patens using in vivo homologous recombination that allows multiple DNA fragments to be transformed at once into the genome (King et al., 2016; Khairul Ikram et al., 2017; Ikram et al., 2019). Here, we describe the techniques employed toward the stable production of artemisinin using P. patens as a heterologous host. P. patens culture preparation and maintenance, transformation technique, and metabolite analysis of artemisinin and intermediate compounds are provided. The graphical representation of the overall procedure is presented in Figure 2.
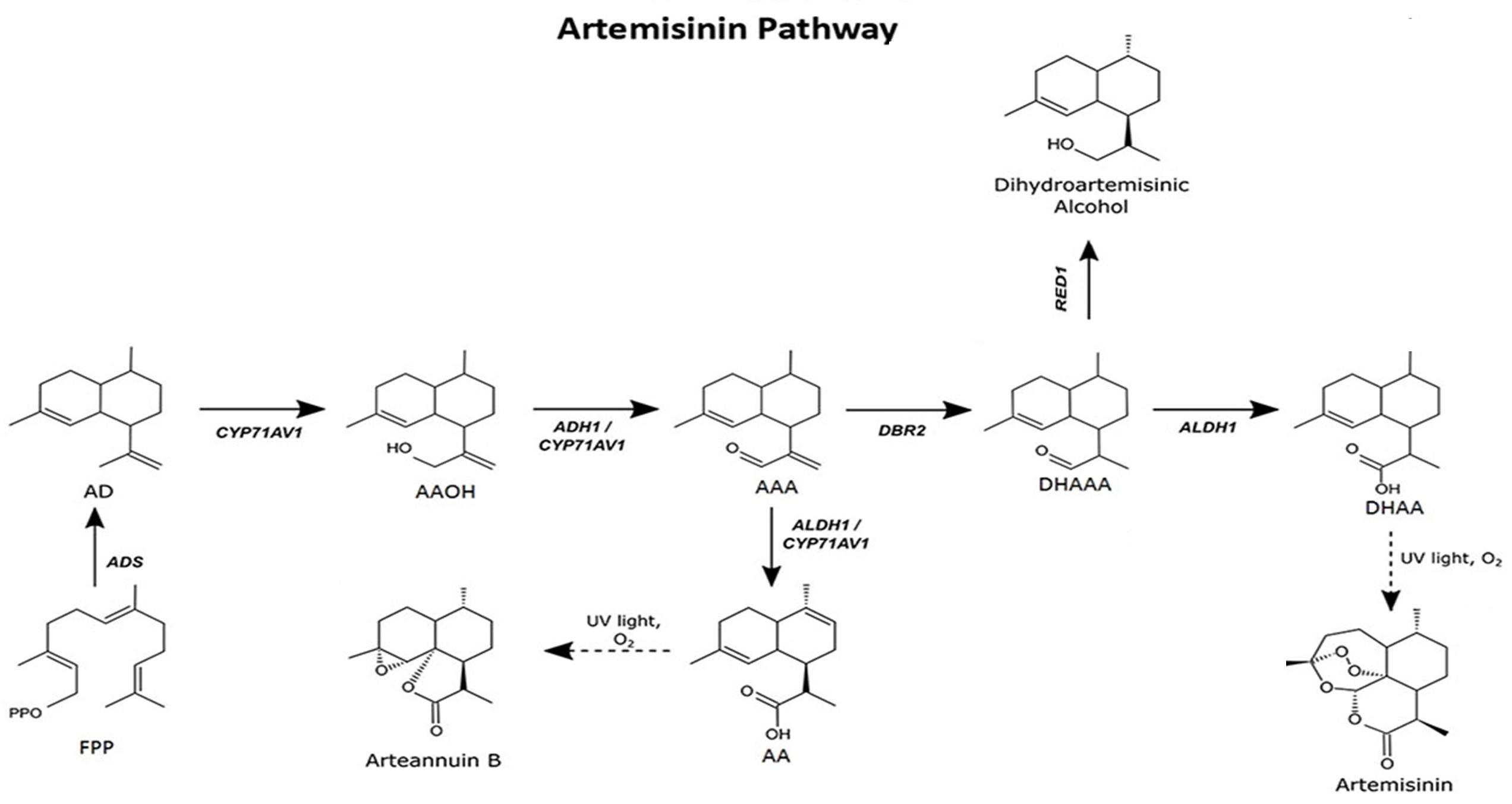
Figure 1. Artemisinin biosynthesis pathway occurs in the glandular trichomes of Artemisia annua. The pathway intermediates are defined as: FPP, farnesyl diphosphate; AD, amorpha-4,11-diene; AAOH, artemisinic alcohol; AAA, artemisinic aldehyde; AA, artemisinic acid; DHAAA, dihydroartemisinic aldehyde; DHAA, dihydroartemisinic acid. Figure adapted from Ikram et al. (2019).
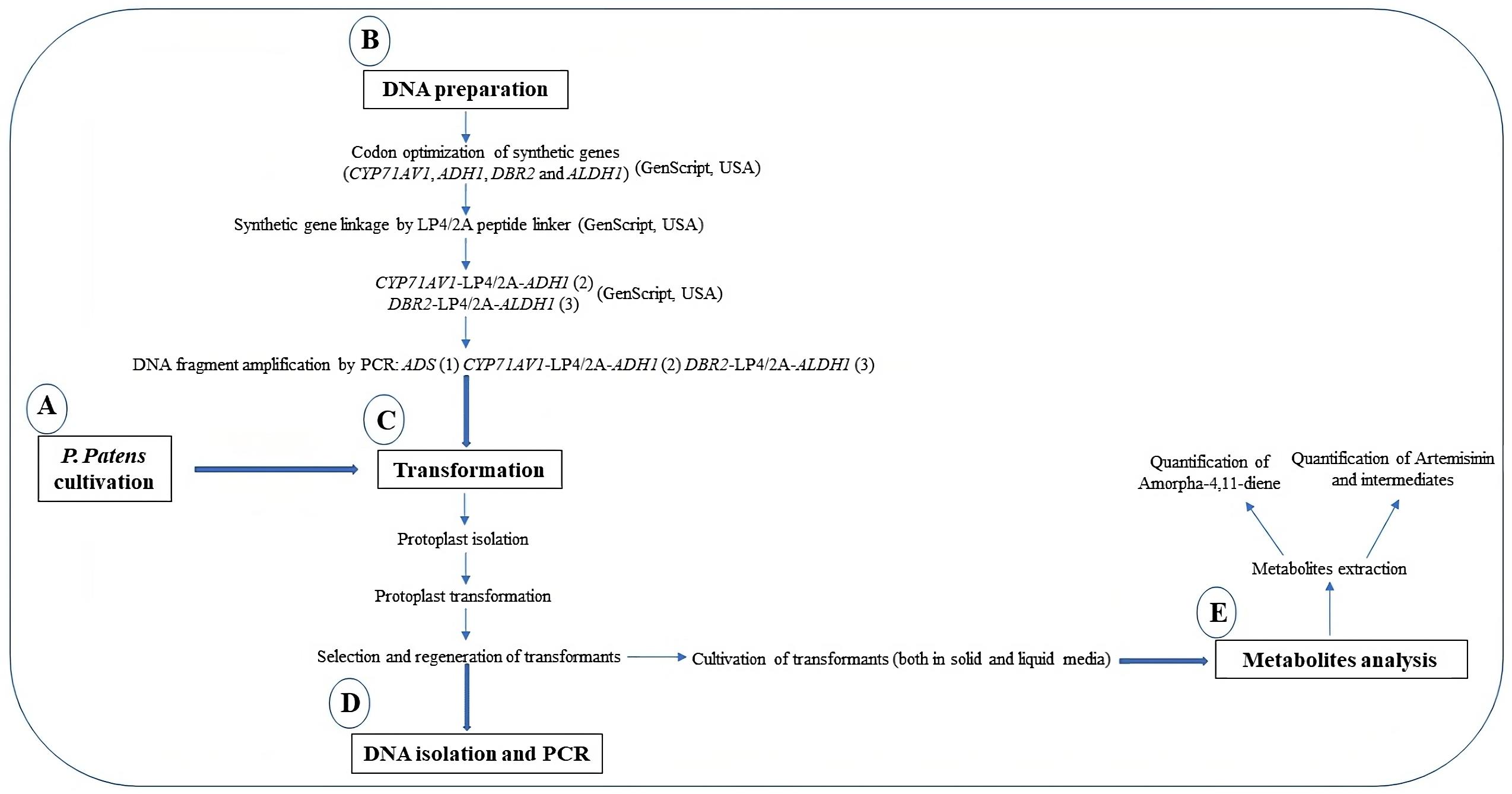
Figure 2. Graphical representation of the protocols
Materials and reagents
Cultivation of Physcomitrium patens
Wildtype Physcomitrium patens (Gransden ecotype #40001) can be obtained from the International Moss Stock Centre at the University of Freiburg, Germany (https://www.moss-stock-center.org/).
Growth room or growth chamber with standard growth condition of a continuous light source with light intensities of 20–50 W/m2 and temperature of 25 °C.
Petri dishes, 90 mm
Serological pipette (Eppendorf)
Sterile pipette (5–50 mL)
Forceps
Note: Wrap forceps separately in foil and autoclave at 121 °C for 20 min.
Cellophane discs
Note: Interleave cellophane discs with filter paper, wrap them in foil paper, and autoclave at 121 °C for 20 min.
Falcon tubes, 50 mL (Thermo Fisher)
Distilled water
Aluminum foil
Erlenmeyer flasks, 50–250 mL
3M surgical tape, 1.25 cm (Micropore, 3M 1533-0)
Tube rack
Parafilm
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C5670-500G)
Ca(NO3)2·4H2O (Sigma-Aldrich, catalog number: C4955-500G)
MgSO4·7H2O (Sigma-Aldrich, catalog number: 63138-1KG)
FeSO4·7H2O (Sigma-Aldrich, catalog number: 215422-250G)
(NH4)2C4H4O6 (Sigma-Aldrich, catalog number: 09985-1KG)
KH2PO4 (Sigma-Aldrich, catalog number: 229806-250G)
KOH (Sigma-Aldrich, catalog number: 60377-1KG)
CuSO4·5H2O (Sigma-Aldrich, catalog number: C3036-250G)
ZnSO4·7H2O (Sigma-Aldrich, catalog number: 221376-500G)
H3BO3 (Sigma-Aldrich, catalog number: B0394-500G)
MnCl2·4H2O (Sigma-Aldrich, catalog number: M3634-500G)
CoCl2·6H2O (Sigma-Aldrich, catalog number: 31277-100G)
KI (Sigma-Aldrich, catalog number: 221945-5G)
Na2MoO4·2H2O (Sigma-Aldrich, catalog number: M1003-100G)
Agar (Duchefa, catalog number: G1101-0500G)
MES (Sigma-Aldrich, catalog number: M3671)
PhyB medium (see Recipes)
1 M CaCl2 (see Recipes)
DNA preparation for Physcomitrium patens transformation
ADS (a gift from Professor Dae Kyun Ro, University of Calgary, Canada)
CYP71AV1 (DQ268763) (GenScript)
ADH1 (JF910157.1) (GenScript)
DBR2 (EU704257.1) (GenScript)
ALDH1 (FJ809784.1) (GenScript)
LP4/2A peptide linker (GenScript)
Ubiquitin promoter from Arabidopsis thaliana (CP002686.1)
Ubiquitin terminator from Arabidopsis thaliana (CP002686.1)
Maize Ubiquitin 1 promoter from the pMP1355 vector
G418 selection cassettes from the pMP1355 vector
Rice actin promoter from the pZAG1 vector
Hygromycin selection cassette from the pZAG1 vector
Microfuge tubes (1.5 mL)
Phusion® High Fidelity DNA Polymerase (New England Biolabs, catalog number: M0530L)
DpnI (New England Biolabs, catalog number: R0176S)
Primers (IDT, USA)
QIAquick PCR Purification kit (QIAGEN GmbH, catalog number: 28104)
Physcomitrium patens transformation
Falcon tubes, 15 mL (Thermo Fisher, catalog number: 14-959-53A)
Serological pipette (Eppendorf, catalog number: 0030127714)
Sterile pipette (5–50 mL)
Cell strainer (100 μm pore size mesh) (Sigma-Aldrich, catalog number: CLS352360)
P. patens culture (five days old)
CaCl2 (Sigma-Aldrich, catalog number: C5670-500G)
MgCl2·6H2O (Sigma-Aldrich, catalog number: M2670-500G)
Driselase® enzyme (Sigma-Aldrich, catalog number: D9515-1G)
D-Mannitol (Sigma-Aldrich, catalog number: M4125-100G)
Tris-Cl (Sigma-Aldrich, catalog number: 10812846001-500G)
Polyethylene glycol (PEG) (MW 6000) solution (Sigma-Aldrich, catalog number: 14504-1G-F)
8.5% D-Mannitol (see Recipes)
Driselase® enzyme solution (see Recipes)
Protoplast wash (PW) solution (see Recipes)
MMM solution (see Recipes)
MCT solution (see Recipes)
Polyethylene glycol (PEG) solution (see Recipes)
Protoplast regeneration medium (bottom layer; PRMB) (see Recipes)
Protoplast regeneration medium (top layer; PRMT) (see Recipes)
DNA isolation and PCR
Microfuge tubes (1.5 mL)
Mortar and pestle
Liquid nitrogen
10% SDS (w/v)
3 M sodium acetate (pH 5.2)
EDTA (Sigma-Aldrich, catalog number: E9884-100G)
Tris (Sigma-Aldrich, catalog number: 10708976001-1KG)
NaCl (Sigma-Aldrich, catalog number: S9888-500G)
Ice
Sea sand (Sigma-Aldrich, catalog number: 1.07711)
Isopropanol (Sigma-Aldrich, catalog number: 563935-1L)
Ethanol (Sigma-Aldrich, catalog number: 459836-2L)
Extraction buffer (see Recipes)
Metabolite analysis
Membrane filter (0.45 μm) (Sartorius, Minisart® RC4)
HP-5MS UI column (20.0 m × 0.18 mm × 0.18 μm) for GC-MS (Agilent)
BEHC18 column (100 mm × 2.1 mm × 1.7 μm) UPLC-MS (Waters)
Artemisinin (Dafra Pharma, Belgium)
Decane (Sigma-Aldrich, catalog number: D901-500ML)
Methanol (Sigma-Aldrich, catalog number: 34860-2.5L)
Acetonitrile (Sigma-Aldrich, catalog number: 34851-2.5L)
Formic acid (Sigma-Aldrich, catalog number: F0507-1ML)
Distilled water
Trans-caryophyllene (Sigma-Aldrich, catalog number: 22075-5ML)
Ethyl acetate (Sigma-Aldrich, catalog number: 270989-2L)
Citrate phosphate buffer (Sigma-Aldrich, catalog number: P4809-50TAB)
Viscozyme® (Sigma-Aldrich, catalog number: V2010-50ML)
Filter paper (Whatman® qualitative filter paper, Grade 1) (Sigma-Aldrich, catalog number: WHA1001325)
Separation funnel
75% (v/v) methanol:water
Acetonitrile:water with formic acid [1:1,000 (v/v)]
Dihydroartemisinin (Dafra Pharma, Belgium)
Artemisinic acid (Chiralix, Nijmegen)*
Artemisinic alcohol (Chiralix, Nijmegen)*
Dihydroartemisinic alcohol (Chiralix, Nijmegen)*
*Synthesized from dihydroartemisinic acid by Chiralix (Nijmegen)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Khairul Ikram, N. K., Zakariya, A. M., Saiman, M. Z., Kashkooli, A. B. and Simonsen, H. T. (2023). Heterologous Production of Artemisinin in Physcomitrium patens by Direct in vivo Assembly of Multiple DNA Fragments. Bio-protocol 13(14): e4719. DOI: 10.21769/BioProtoc.4719.
分类
生物工程 > 合成生物学 > 基因修饰
植物科学 > 植物新陈代谢 > 其它化合物
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










