- EN - English
- CN - 中文
Selection of Molecules with Immunological Potential from Excretory and Secretory Products from the Nematode Haemonchus placei by Cell Proliferation and Gene Expression Assays
用细胞增殖和基因表达测定法从柏氏血矛线虫排泄物和分泌物中筛选具有免疫潜力的分子
发布: 2023年06月20日第13卷第12期 DOI: 10.21769/BioProtoc.4702 浏览次数: 1329
评审: Jorge Francisco GonzálezAnonymous reviewer(s)
Abstract
The nematode Haemonchus placei is a pathogenic parasite, the most seriously affecting ruminant’s health and being responsible for enormous economic losses all over the world. The present protocol describes different in vitro techniques to select potential candidate antigens with immune-protective activity from excretory and secretory products (ESP) from H. placei transitory infective larvae (xL3). ESP from xL3 were obtained from the in vitro infective larvae (L3) maintained in Hank’s medium at 37 °C with 5% CO2 for 48 h. Then, the presence of ESP proteins was confirmed by SDS-PAGE to be used in an in vitro proliferation assay with bovine peripheral blood mononuclear cells (PBMCs). The ESP were exposed to the PBMCs during two different periods (24 and 48 h). Genes associated with immune response against the nematode were analyzed using relative gene expression and bioinformatic tools. These are simple, economic, and helpful tools to identify potential immune-protective molecules under in vitro conditions for confirming the efficacy of future in vivo assays.
Graphical overview
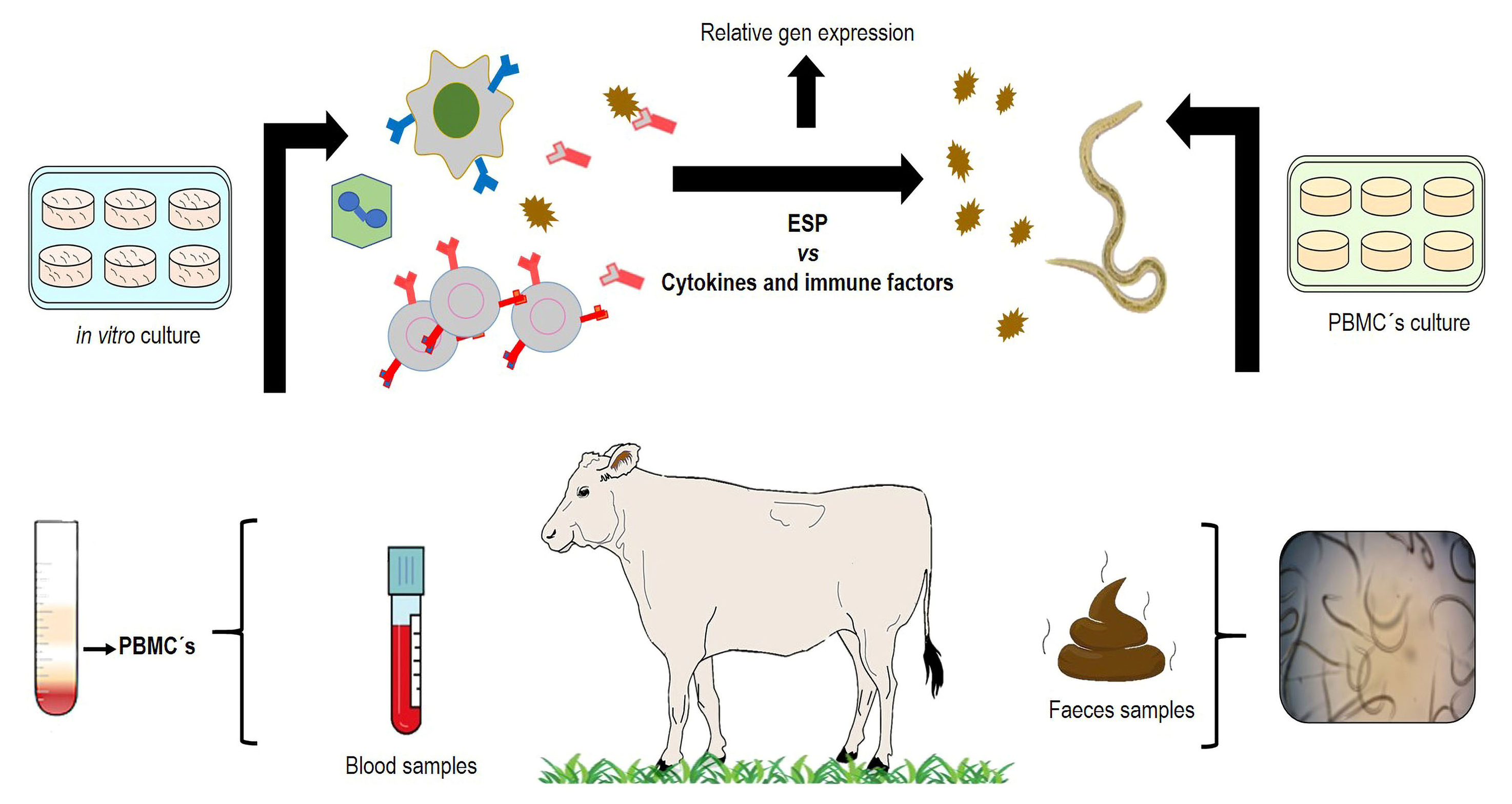
Background
Domestic ruminants are severely affected by the complex of gastrointestinal nematodes (GINs). Some parasites belonging to this group (i.e., Ostertagia and Haemonchus) are particularly highly pathogenic due to their blood-feeding habits. The genus Haemonchus spp. is the most pathogenic parasitic nematode, particularly in tropical regions where its prevalence reaches > 80% (Liébano-Hernández et al., 2011). Traditionally, the control of GINs is based on the use of anthelmintic drugs; however, anthelmintic resistance has dramatically increased during the last decades, risking animal health and production. In order to reduce the use of chemical anthelmintic drugs, different strategies of control and prevention have been explored. One of them is assessing the use of specific proteins as potential immunizing agents, mainly those that are involved in the host–parasite interaction. The genus Haemonchus has been used as a biological model for understanding the invasive process to the host tissues. During invasion of the gastric mucosa tissues, different excretory and secretory products (ESP), mainly proteins, are produced; some of them could have important implications as potential immune protective antigens. The in vitro development from Haemonchus spp. infective larvae (L3) to the transitory infective larvae (xL3) is an easy and economic technique that allows the release of potential antigenic molecules with important immunoprotective value. The invasion mechanism of larvae to the host tissue and their further development require the activity of enzymes to use nutrients and thus achieve the adult stage for surviving in the abomasum (stomach) of ruminants. Recently, numerous studies have intensified the identification of ESP to induce immune protection, thus hampering the larvae’s invasive process. In addition, the importance of ESP during tissue invasion by parasitic nematodes is associated to inflammatory and regulatory immune mechanisms such as cytokines (e.g., IL4, IL5, IL6, IL8, IL10), immunoglobulins (IgA, IgG, and IgE), and other important immune cells like eosinophils and neutrophils. The study of antigens and immune cells requires the use of different experimental in vitro and in vivo processes, to improve the identification of ESP with possible biological functions. The present methodology describes reproducible experimental procedures for obtaining and studying the nematode’s ESP and the host’s immune response. The following protocol describes methods related to parasitology, immunology, and molecular procedures to obtain ESP from xL3of the nematode speciesHaemonchus placeiand to evaluate them in vitro activity.
Materials and reagents
T-75 cell culture flask (Thermo Fisher, catalog number: 156800)
Gauze (any brand can be used)
McMaster egg counting chamber (JorVetTM, catalog number: J0335M)
Plastic container, 18 cm × 38 cm (any brand can be used)
Foam rubber (any brand can be used)
Aluminum foil (any brand can be used)
Distilled water (any brand can be used)
Polypropylene funnel 150 mm diameter (Corning®, catalog number: 6120P-150)
10 mL culture tube (Pyrex®, catalog number: 99445-13)
Plastic hose assembly (Fisher Scientific, catalog number: 02-594-1F)
Pasteur plastic pipette, 1 mL (MediLab, catalog number: 121227/01)
Optical lens wipes (Carolina Biol. Supply Co., catalog number: 634000)
15 mL plastic centrifuge tube (Corning®, catalog number: 430052)
50 mL plastic centrifuge tube (Corning®, catalog number: 430828)
6-well plate, flat-bottom, clear and sterile, TC-treated (Corning®, catalog number: 3506)
Petri dishes 100 mm × 15 mm, sterile (Falcon®, catalog number: 351029)
Diameter syringe filters 0.2 μm pore (Corning®, Catalog number: 431219)
Vacutainer tubes with EDTA (Becton Dickinson, catalog number: 367863)
Hypodermic green needles, 21 G × 32 mm (Nipro®, catalog number: A21)
Pasteur pipettes (Sigma, catalog number: S5893)
0.2 mL PCR Tubes with flat cap (Axygen®, catalog number: PCR-02-C)
96 well-microplates, flat-bottom, clear and sterile, TC-treated (Corning®, catalog number: 3628)
24-well plate, flat-bottom, clear and sterile, TC-treated (Corning®, catalog number: 3527)
1.5 mL microcentrifuge plastic tuber (Axygen®, catalog number: MCT-150-A)
Plastic pestle (Bel-ArtTM, catalog number: F19923-0001)
Recently collected fecal samples from infected cattle withH. placei(300–500 g)
Sodium chloride (NaCl) of commercial food grade (any brand can be used)
Sucrose (Sigma-Aldrich, catalog number: MFCD00006626)
Sodium hypochlorite (NaClO) (Cloralex-Grupo AlEn-México)
Antibiotic-antimycotic (100×) (Thermo Fisher Scientific, GibcoTM, catalog number: 15240062)
Hanks’ Balanced Salts without sodium bicarbonate (Sigma, catalog number: H6136)
Ethanol molecular grade (Hycel, catalog number: 1822-500)
Peripheral blood sample from three non-infected cattle (36 mL)
LymphoprepTM (Alere Technologies, Axis-Shield, catalog number: 1114547)
RPMI 1640 medium with L-glutamine and HEPES (Gibco, catalog number: 23400062)
Fetal bovine serum (FBS) (By Products, catalog number: 90020)
Trypan blue stain (Thermo Fisher Scientific, GibcoTM, catalog number: 15250061)
Neubauer Improved bright line (MARIENFELD, catalog number: 0640011)
CellTiter 96® Aqueous One Solution Cell Proliferation assay (Promega, catalog number: G3582)
QIAzol lysis reagent (Qiagen®, catalog number: 79306)
Chloroform (J.T. Baker, catalog number: 9180)
Isopropyl alcohol (Sigma-Aldrich, catalog number: W292907)
Nuclease-free water (Promega, catalog number: P1193)
Agarose (Bio-Rad, catalog number: 1613100)
Ethidium bromide 10 mg/mL (Bio-Rad, catalog number: 1610433)
RQ1 RNase-Free DNase kit (Promega, catalog number: M6101)
ImProm-II Reverse Transcription System kit (Promega, catalog number: A3800)
GoTaq® qPCR Master Mix 2× (Promega, catalog number: A6001)
Custom RT2 Profiler PCR array (Qiagen, catalog number: CAPB13410R)
Trizma base (Sigma, catalog number: 93362)
Glacial acetic acid (Meyer, catalog number: 0040)
EDTA 0.5 M pH 8.0 (Invitrogen, catalog number: AM9260G)
Potassium chloride (KCl) (Sigma, catalog number: P3911)
Sodium phosphate dibasic (Na2HPO4) (Sigma, catalog number: RDD038)
Potassium phosphate monobasic (KH2PO4) (Sigma, catalog number: 221309)
1× sterile phosphate buffered saline (PBS), pH 7.4 (see Recipes)
Buffer 50× TAE (see Recipes)
3% agarose gel (see Recipes)
0.187% NaClO solution (see Recipes)
Hank’s balanced salts (medium) (see Recipes)
40% sucrose solution (see Recipes)
Saturated sodium chloride solution (see Recipes)
Equipment
Centrifuge (Thermo Fisher Scientific, Sorvall ST 8R, catalog number: 75007204)
Microscopy (Zeizz, Primo Star Hal/Led Full-Köhler ERc5s, catalog number: 415500-0057-000)
0.1–10 μL, 2–20 μL, 20–200 μL, and 100–1,000 μL micropipettes (any brand can be used)
Shaker (Labnet, OrbitTM 1000, catalog number: S2030-1000-B)
Incubator (ECOSHEL, CI-80, CO2incubator)
Mixer (Benchmark Scientific, VortexTM, catalog number: BV101-P)
Microplate absorbance reader (Bio-Rad, iMarkTM, catalog number: 1681135)
Nanophotometer (Implen, NP80)
PowerPAc chamber (Bio-Rad, 300, catalog number: 165-5050)
Thermal cycler 6000 (Qiagen, Corbett Rotor-Gene 6000)
Transilluminator Imagine System (UVP, EC3, catalog number: 81-020901)
Nucleic acid electrophoresis chamber (any brand can be used)
Granatary scale 610 × 0.1 g (any brand can be used)
Freezer of -80 °C (any brand can be used)
pH meter (any brand can be used)
Software
Rotor-Gene Q—Pure detection Software (version 1.7)
GeneGlobe Data Analysis Center of Qiagen® (https://geneglobe.qiagen.com/analyze/)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Maza-Lopez, J., Camas-Pereyra, R., López-Arellano, M. E. and Contreras-Ochoa, C. O. (2023). Selection of Molecules with Immunological Potential from Excretory and Secretory Products from the Nematode Haemonchus placei by Cell Proliferation and Gene Expression Assays. Bio-protocol 13(12): e4702. DOI: 10.21769/BioProtoc.4702.
分类
免疫学 > 免疫细胞分离 > 白细胞
医学
分子生物学 > RNA > qRT-PCR
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














