- EN - English
- CN - 中文
Isolation and Quantification of Mandelonitrile from Arabidopsis thaliana Using Gas Chromatography/Mass Spectrometry
气相色谱/质谱法分离和定量分析拟南芥中的扁桃腈
发布: 2023年06月20日第13卷第12期 DOI: 10.21769/BioProtoc.4700 浏览次数: 1574
评审: Xiaofei LiangPrajita PandeySneha RayAnonymous reviewer(s)
Abstract
Mandelonitrile is a nitrogen-containing compound, considered an essential secondary metabolite. Chemically, it is a cyanohydrin derivative of benzaldehyde, with relevant functions in different physiological processes including defense against phytophagous arthropods. So far, procedures for detecting mandelonitrile have been effectively applied in cyanogenic plant species such as Prunus spp. Nevertheless, its presence in Arabidopsis thaliana, considered a non-cyanogenic species, has never been determined. Here, we report the development of an accurate protocol for mandelonitrile quantification in A. thaliana within the context of A. thaliana–spider mite interaction. First, mandelonitrile was isolated from Arabidopsis rosettes using methanol; then, it was derivatized by silylation to enhance detection and, finally, it was quantified using gas chromatography–mass spectrometry. The selectivity and sensitivity of this method make it possible to detect low levels of mandelonitrile (LOD 3 ppm) in a plant species considered non-cyanogenic that, therefore, will have little to no cyanogenic compounds, using a small quantity of starting material (≥100 mg).
Keywords: Benzaldehyde cyanohydrin (苯甲醛氰醇)Background
Cyanogenic glycosides (CNglcs) are secondary plant metabolites arisen from amino acids and found in over 3,000 plant species, including economically important crops as Prunus spp., Manihot spp., Malus spp., or Sorghum spp. (Gleadow and Møller, 2014). CNglcs play crucial roles in different physiological processes such as germination, transport of essential nutrients, turnover processes, oxidative stress regulation, or defense responses (Møller, 2010; Kadow et al., 2012; Flematti et al., 2013; Neilson et al., 2013). The metabolism of CNglcs is complex and derives in cyanohydrin production, which in turn can be converted into hydrogen cyanide and ketones or aldehydes in the presence of α-hydroxynitrile lyase enzymes (HNLs). Plant cyanohydrins have been demonstrated to act as direct defense molecules, with a highly toxic action against herbivores and pathogens (Park et al., 2002; Beran et al., 2019). In addition, they can also work as signaling molecules, triggering the defense response (Bernal-Vicente et al., 2020). However, some herbivores, mainly lepidopteran insects, have developed the ability to counteract or take advantage of these cyanogenic molecules, and alternatively may degrade or use these compounds for their benefit (Zagrobelny and Møller, 2011; Zagrobelny et al., 2014).
The focus of this protocol is one of these CNglcs derivatives, mandelonitrile. Mandelonitrile is a cyanohydrin derived from CNglc metabolism, as prunasin or amygdalin among others (Gleadow and Møller, 2014), that occurs naturally in cyanogenic plants. It is characterized by having hydroxyl and cyano groups attached to the same carbon atom (Figure 1). Some works have reported the quantification of mandelonitrile in different cyanogenic plant species such as Prunus cerasus L. and Aronia melanocarpa Ell using high-performance liquid chromatography (HPLC) and gas chromatography–mass spectrometry (GC/MS) techniques, respectively (Hirvi and Honkanen, 1985; Chandra and Nair, 1993).
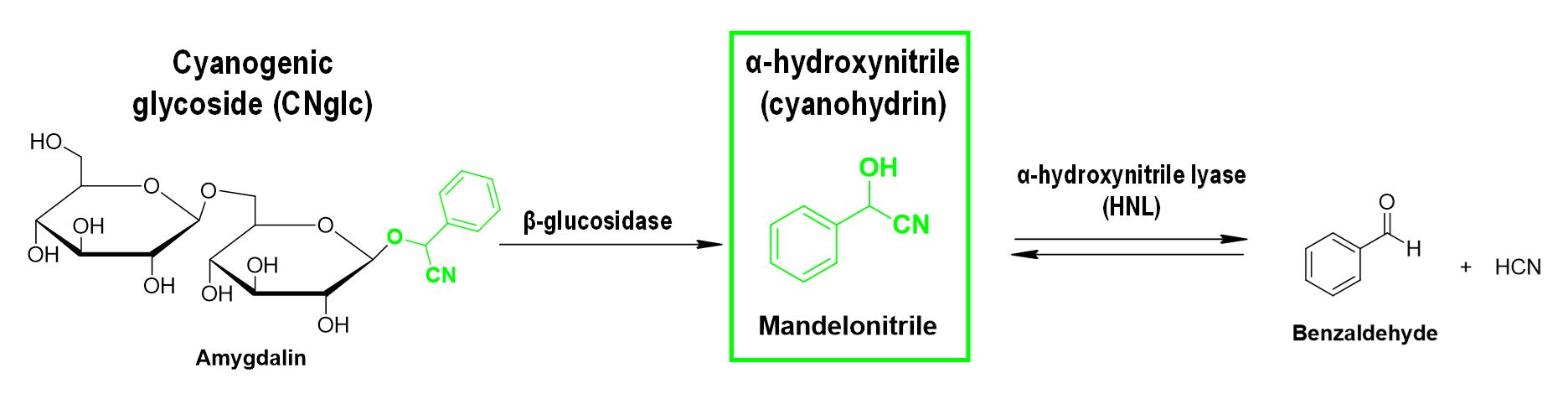
Figure 1. Example of a cyanogenesis pathway in plants with mandelonitrile as the central player. HCN: hydrogen cyanide.
Chassagne et al. (1996) detected amygdalin and its corresponding parental ion, benzaldehyde cyanohydrin (or mandelonitrile), in Passiflora fruits using GC/MS. More recently, mandelonitrile has been quantified in peach micro-propagated shoots by ultra-performance liquid chromatography (UPLC) coupled to a mass spectrophotometer (Bernal-Vicente et al., 2020). However, as far as we know, mandelonitrile levels haven’t been determined in the model plant A. thaliana until now, maybe because it is considered a non-cyanogenic plant (CNglcs have not been detected in A. thaliana wild-type plants). Despite this, an alternative pathway to cyanohydrin production has recently been described—the 4-hydroxy-indole-3-carbonylnitrile (4-OH-ICN) route—as a rare metabolic re-invention leading to alternative cyanogenic compounds, to expand A. thaliana defenses and also contribute to the production of cyanohydrins (Rajniak et al., 2015; Pastorczyk et al., 2020). In addition, an HNL has also been found in A. thaliana, and its encoding gene has been cloned and the recombinant protein has been purified and crystalized (Andexer et al., 2007). Biochemical studies revealed that Arabidopsis HNL recognized a broad range of substrates, was enantioselective, and transformed aliphatic and aromatic aldehydes and/or ketones into the corresponding R-cyanohydrins, including mandelonitrile (Andexer et al., 2007).
Therefore, the study of the relationship between mandelonitrile levels and the role of HNL in Arabidopsis has allowed the establishment of their defense role against the two-spotted spider mite Tetranychus urticae (Arnaiz et al., 2022). Consequently, mandelonitrile can be considered a target molecule for the study of Arabidopsis–spider mite interaction in the plant defense process. This is why the development of a protocol to determine the levels of mandelonitrile in A. thaliana plants under controlled conditions and after spider mite infestation is of crucial interest. In addition, this protocol was established since it was not possible to adapt previous protocols already described for other plant species. In fact, no results were obtained using HPLC techniques and with the available quantity of infested A. thaliana sample as starting plant material, suggesting the need to develop a specific quantification procedure for such a non-cyanogenic species. GC/MS was selected for mandelonitrile detection as it allowed to detect the target molecule in standard samples. Moreover, the spectrum information related to mandelonitrile-derivatized found in the databases helped us to confirm our obtained results. Additionally, the derivatization process of mandelonitrile makes it a very volatile molecule and for that reason, it was better to use GC/MS than HPLC for analysis. Finally, it is likely that this protocol will work in other plant species by applying some modifications, e.g., quantity of starting material, solvent volume, or incubation and sonication times.
Materials and reagents
Biological material
Arabidopsis thaliana Columbia-0 (Col-0) ecotype seeds were purchased at the Arabidopsis Biological Resource Center
Tetranychus urticae, London strain (Acari: Tetranychidae). Dr. Miodrag Grbic (UWO, Canada) provided the spider mite colony (see Note 1)
Materials
Microcentrifuge tube with safe lock 1.5 mL (Astik’s, catalog number: PCRP-015-500)
Pipette tips (Astik’s, catalog numbers: TIPP-1K0-10K, TIPP-200-10K, and TIPP-010-10K)
Peat moss (Klasmann-Deilmann, catalog number: L1070K0600)
Vermiculite (Valimex S.L., catalog number: 100M1006)
Plastic alveolus tray (Projar, catalog number: 4400000S28)
Cling film (KINGSWAY, catalog number: KING756046)
Aluminum foil (Alujet-Universal, catalog number: 293-4176)
Mortar and pestle (LGB, catalog number: MORN-075-001)
Tweezers (Clink, catalog number: FORS-003-002)
Micro spatula (Clink, catalog number: SPNS-150-005)
Centrifuge tubes with screw cap, 15 mL (Astik’s, catalog number: PTGP-E15-025)
Crimp neck vials ND11 (VWR, catalog number: 548-8008A)
Crimp caps with septa for crimp neck vials ND11 (VWR, catalog number: 548-8004A)
Minisart® syringe filters PTFE 0.2 μm (Sartorius, catalog number: 17573-ACK)
Gas chromatography column DB-5MS UI (Agilent, catalog number: 122-5562UI)
Chemicals and solvents
Sodium dodecyl sulfate (SDS) (PanReac AppliChem ITW Reagents, catalog number: A2263.0100)
Sodium hypochlorite (PanReac AppliChem ITW Reagents, catalog number: 212297.1211)
Methanol (VWR, catalog number: 20864.32)
Mandelonitrile (Sigma-Aldrich, catalog number: 116025)
Ethanol absolute (VWR, catalog number: 20821.365)
Acetonitrile (VWR, catalog number: 83639.320)
2,2,2-Trifluoro-N-methyl-N-(trimethylsilyl)acetamide (MSTFA) (Supelco, catalog number: 1.11805)
Silicone oil for oil baths (VWR, catalog number: 24610.363)
Autoclaved distilled water
Arabidopsis seeds sterilization solution (see Recipes)
Equipment
Single-channel pipettes PIPETMAN® L P1000 (Gilson, catalog number: FA10006M)
Single-channel pipettes PIPETMAN® L P200 (Gilson, catalog number: FA10005M)
Single-channel pipettes PIPETMAN® L P20 (Gilson, catalog number: FA10003M)
Single-channel pipettes PIPETMAN® L P2 (Gilson, catalog number: FA10001M)
Elmasonic S30H (Elma Schmidbauer GmbH, catalog number: 1001955)
Heating oven Binder E028 (BINDER GmbH, catalog number: 9010-0001)
Sorvall X4F Pro centrifuge (Thermo Scientific, catalog number: 75009026)
Digital rotary evaporator (VWR, catalog number: 531-1365P)
Precision balance LPW 2103i (VWR, catalog number: 611-3283)
Agilent 7890B gas chromatography system coupled to an Agilent 7010 triple quadrupole mass spectrometer (Agilent)
Freezer -80 °C
Sanyo MLR-350-H Panasonic growth chamber
Laminar flow hood
4 °C chamber
Software
MassHunter Workstation software version B.07.01 (Agilent)
Microsoft office Excel
Statistical analysis software such as GraphPad Prism 9, OriginPro 9.0, or Statgraphics
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Arnaiz, A., Vallejo-García, J. L., Vallejos, S. and Diaz, I. (2023). Isolation and Quantification of Mandelonitrile from Arabidopsis thaliana Using Gas Chromatography/Mass Spectrometry. Bio-protocol 13(12): e4700. DOI: 10.21769/BioProtoc.4700.
分类
环境生物学 > 植物 > 植物-昆虫互作
植物科学 > 植物分子生物学
分子生物学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













