- EN - English
- CN - 中文
Synthesis of Bacteria-mimetic Gold Nanoparticles for Phagocytosis by Immune Cells
用于免疫细胞吞噬的仿生细菌金纳米粒子的合成
(*contributed equally to this work) 发布: 2023年06月20日第13卷第12期 DOI: 10.21769/BioProtoc.4695 浏览次数: 2388
评审: Alka MehraSameer NadafChhuttan L MeenaKarem A Court
Abstract
Cell-based carrier exhibits inherent advantages as the next generation of drug delivery system, namely high biocompatibility and physiological function. Current cell-based carriers are constructed via direct payload internalization or conjugation between cell and payload. However, the cells involved in these strategies must be firstly extracted from the body and the cell-based carrier must be prepared in vitro. Herein, we synthesize bacteria-mimetic gold nanoparticles (GNPs) for the construction of cell-based carrier in mice. Both β-cyclodextrin (β-CD)-modified GNPs and adamantane (ADA)-modified GNPs are coated by E. coli outer membrane vesicles (OMVs). The E. coli OMVs induce the phagocytosis of GNPs by circulating immune cells, leading to intracellular degradation of OMVs and subsequent supramolecular self-assembly of GNPs driven by β-CD-ADA host–guest interactions. In vivo construction of cell-based carrier based on bacteria-mimetic GNPs avoids the immunogenicity induced by allogeneic cells and restriction by the number of separated cells. Due to the inflammatory tropism, endogenous immune cells carry the intracellular GNP aggregates to the tumor tissues in vivo.
Graphical overview
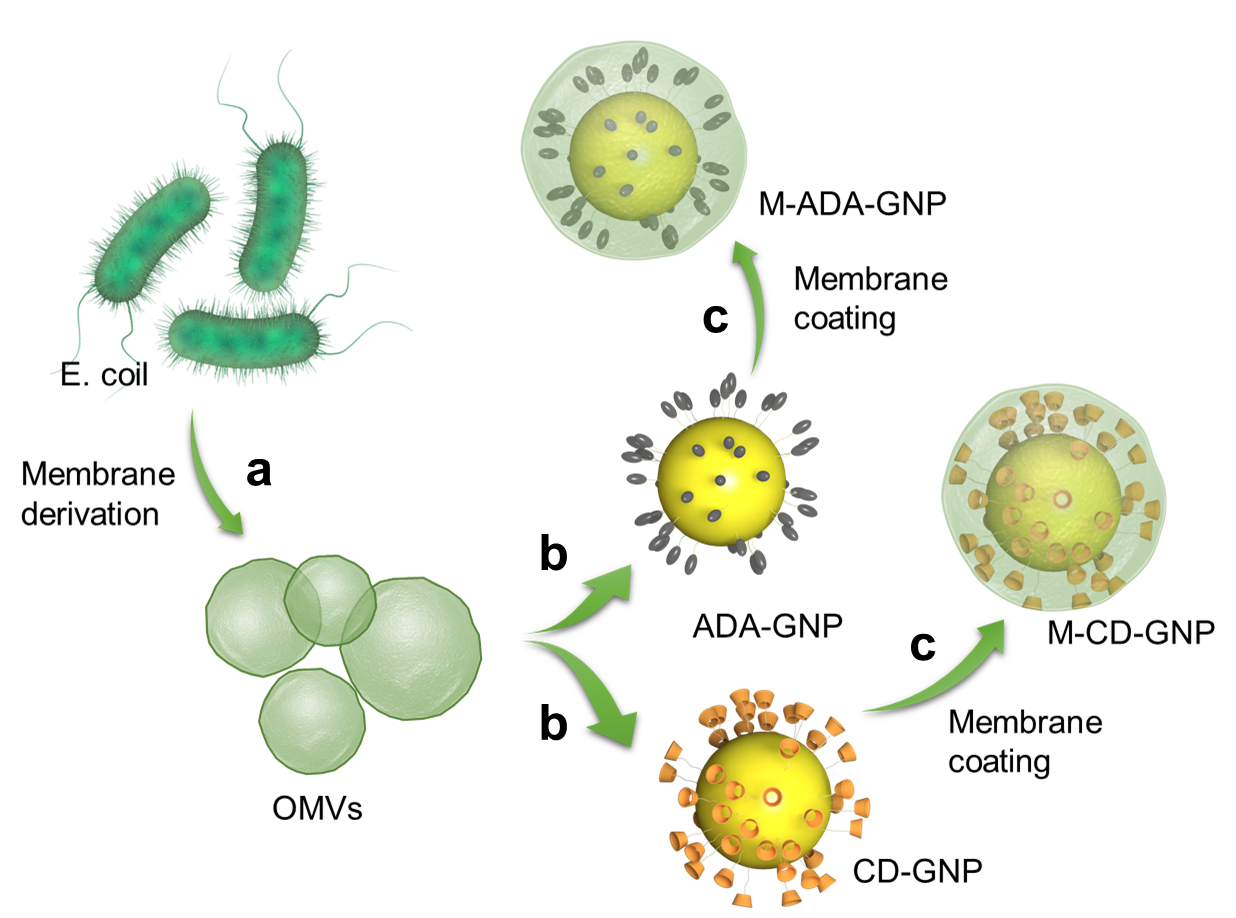
Collect the outer membrane vesicles (OMVs) of E. coli by gradient centrifugation (a) and coat on gold nanoparticles (GNP) surface (b) to prepare OMV-coated cyclodextrin (CD)-GNPs and OMV-coated adamantane (ADA)-GNPs (c) via ultrasonic method
Background
As a self component, cells exhibit inherent physiological properties in drug delivery, such as biocompatibility and homing effect (Yang et al., 2022). These help the drug payload to escape clearance by the mononuclear phagocyte system and perform the physiological function–based hitchhiking delivery. Recently, cell-based carriers were mainly constructed via direct drug internalization; this procedure was very simple, needing only in vitro cell culture with the payload (Li et al., 2021). In addition, another construction strategy was surface conjugation between cell and payload, which could be divided into covalent conjugation, receptor–ligand interaction, host–guest interaction, and physical interaction (Krueger et al., 2018; Li et al., 2018). The host cell must be artificially modified in vitro and then conjugated with payload. Thus, cell-based carriers, via either intracellular drug internalization or cell-surface attachment, were constructed in vitro before in vivo administration. This presented three challenges in clinical translation and application: 1) large-scale preparation is barely possible due to the limitation of separating sufficient number of endogenous cells; 2) in vitro drug loading processes may inevitably affect the physiological function of the carrier cells; and 3) transporting cells correspond to one single host, and would generate immune rejection when applied to other individuals.
In light of these issues, bacteria-mimetic gold nanoparticles (GNPs) were developed for in vivo construction of immune cell–based carriers and efficiently and stably hitchhiking delivery. In this design, both β-cyclodextrin (β-CD)-modified GNPs (CD-GNPs) and adamantane (ADA)-modified GNPs (ADA-GNPs) are coated by E. coli outer membrane vesicles (OMVs). Host–guest interactions involve two molecules or materials that can form complexes through unique structural relationships and noncovalent binding. Due to host–guest interactions between β-CD and ADA (Loescher and Walther, 2020; Wang et al., 2021), the host β-CD could bind with guest ADA to form a supramolecular complex. Thus, CD-GNPs bound with ADA-GNPs to produce GNP aggregates. It has been observed that the GNP aggregates show higher absorption ratio and therefore, higher temperature rise compared to single GNPs. The effect of photothermal therapy (PTT) was significantly improved in the form of GNP aggregates in comparison to GNPs. The E. coli OMVs coating induce the phagocytosis of GNPs by circulating immune cells, leading to intracellular degradation of OMVs and subsequent supramolecular self-assembly of GNPs. In addition to the increased PTT efficiency, GNP aggregation increased the nano-size of GNP into micro-size of GNP aggregate, which significantly inhibits the leakage of GNPs from immune cells (Zhang et al., 2021). This bacterial OMV-coating strategy for immune cell phagocytosis was not only applied to GNPs, but also extended to other therapeutic reagents that directly modulate immune cells or target disease tissues via immune cell hitchhiking delivery.
As different cells (platelets, stem cells, macrophages, T cells, or even tumor cells) have different physiological functions, the coating material could be specifically designed for phagocytosis according to the required type of cells as carriers for hitchhiking delivery.
Materials and reagents
Falcon tube (Falcon, catalog number: 352098)
Millipore with 10 kDa filter (Millipore, catalog number: UFC901024)
Nylon membrane filter with 0.45 pore size (Sigma-Aldrich, catalog number: Z290815-100EA)
HAuCl4·3H2O (Sigma-Aldrich, catalog number: 520918)
Sodium citrate (Aladdin, catalog number: S189183-100g)
Mono(6-mercapto-6-deoxy)-beta-cyclodextrin (Shandong Binzhou Zhiyuan Biotechnology Co., Ltd, catalog number: 81644-55-5)
1-adamantanethiol (Aladdin, catalog number: A169723-5g)
E. coli (ATCC, catalog number: 33694)
Polypropylene bacterial culture tube (Falcon, catalog number: 352057)
DMSO (Sigma-Aldrich, catalog number: D8418-100ML)
Lysogeny broth (LB) (Thermo Fisher Scientific, catalog number: 10855021)
LB medium (see Recipes)
Sodium citrate solution (5%) (see Recipes)
HAuCl4 solution (0.25 mM) (see Recipes)
Mono(6-mercapto-6-deoxy)-beta-cyclodextrin solution (2 mM) (see Recipes)
GNP solution (10 mM) (see Recipes)
1-adamantanethiol in DMSO (2 mM) (see Recipes)
Equipment
Centrifuge (Thermo Fisher Scientific, Heraeus Multifuge X3, model: 10325804)
Dynamic light scattering (Malvern, Nano-ZS)
Ultra-high-speed centrifuge (Beckman, Optima XPN-100 Ultracentrifuge)
Sonication bath (Branson, Bransonic ultrasonic cleaner, model: 5510E-DTH)
Transmission electron microscope, 120 kV (HITACHI, model: HT7800)
Microplate reader (Molecular Devices, SpectraMax, model: M5)
Vacuum oven (Thermo Fisher Scientific, Vacutherm, model: VT6205)
Software
Software associated with plate reader used (SoftMax Pro 7 Software)
Software associated with dynamic laser scanning used (Malvern Zetasizer Software v7.11)
Software associated with transmission electron microscope used (Gatan DigitalMicrograph 3.9)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Gao, C., Tang, M., Lee, S. M. Y. and Wang, R. (2023). Synthesis of Bacteria-mimetic Gold Nanoparticles for Phagocytosis by Immune Cells. Bio-protocol 13(12): e4695. DOI: 10.21769/BioProtoc.4695.
- Gao, C., Wang, Q., Li, J., Kwong, C. H. T., Wei, J., Xie, B., Lu, S., Lee, S. M. Y. and Wang, R. (2022). In vivo hitchhiking of immune cells by intracellular self-assembly of bacteria-mimetic nanomedicine for targeted therapy of melanoma. Sci Adv 8(19): eabn1805.
分类
细胞生物学 > 细胞器分离 > 细胞膜
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













