- EN - English
- CN - 中文
Synthesis and Purification of Lipid-conjugated Fluorescent pH Sensors
脂质共轭荧光pH传感器的合成与纯化
发布: 2023年06月05日第13卷第11期 DOI: 10.21769/BioProtoc.4694 浏览次数: 1934
评审: Gal HaimovichPhilipp A.M. SchmidpeterAnonymous reviewer(s)
Abstract
Lipid-conjugated pH sensors based on fluorophores coupled to lipids are a powerful tool for monitoring pH gradients in biological microcompartments and reconstituted membrane systems. This protocol describes the synthesis of pH sensors based on amine-reactive pHrodo esters and the amino phospholipid phosphatidylethanolamine. The major features of this sensor include efficient partitioning into membranes and strong fluorescence under acidic conditions. The protocol described here can be used as a template to couple other amine-reactive fluorophores to phosphatidylethanolamines.
Graphical overview
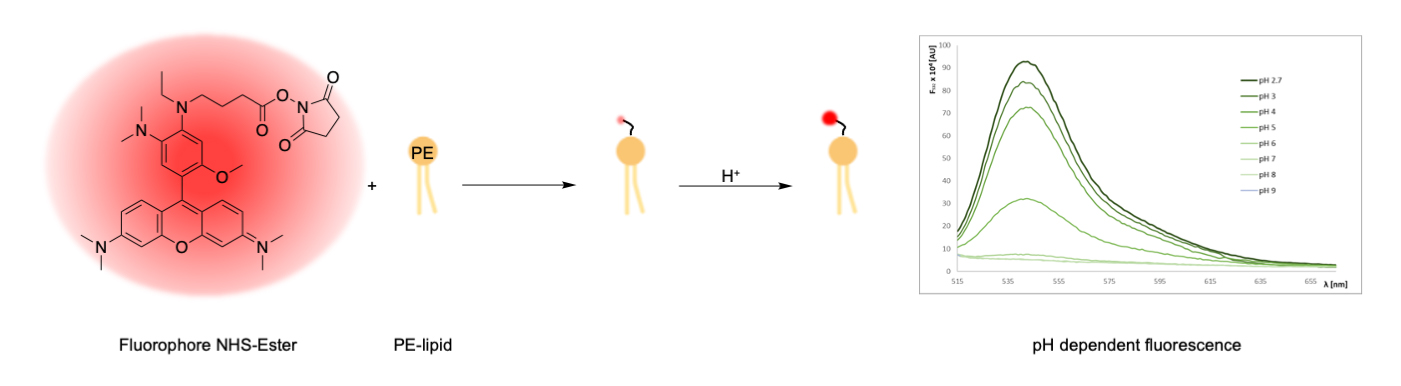
Synthesis of lipid-conjugated pH sensors based on amine-reactive fluorophore esters and the aminophospholipid phosphoethanolamine (PE)
Background
Cells and organelles depend on electrochemical gradients across their membranes, which are maintained through ATP-driven, membrane-embedded transporter proteins. Transport of ions across cellular membranes can be primary or coupled to drive the uptake or export of other solutes. Perturbations of the highly regulated transport systems are the cause to many diseases and can be the reason for failure of pharmacotherapy (Padan and Landau, 2016; Clausen et al., 2017; Picci et al., 2022). Given the pivotal role of these transporters in cell homeostasis and health, a thorough understanding of their organization, functioning, and dynamics is highly desirable. A powerful tool to visualize the activity of ion transporters in living cells and reconstituted membrane systems are fluorescent compounds based on synthetic molecules. Although a variety of ion-targeting probes are commercially available, many have limitations in studies with membrane transporters. For example, water-soluble dyes such as pyranine (8-hydroxypyrene-1,3,6-trisulphonic acid) and fluorescamine (4-phenylspiro-[furan-2(3H),1-phthalan]-3,3′-dion) have been used to monitor the intravesicular pH values in studies of H+-translocating proteins (S. Li et al., 2011; Ohlsson et al., 2012; M. Li et al., 2015; Berg et al., 2017). However, their modest brightness (pyranine) and high bleaching rates (fluorescamine) make them difficult to use for pH measurements in vivo and in vitro. Rhodamine-based dyes provide an alternative due to their improved brightness and photostability; however, for reconstituted systems, problems with insufficient encapsulation during protein reconstitution and leakage out of vesicles were reported (Schubert, 2003; Leiding et al., 2009). More recently, membrane-bound pH sensors based on lipid-conjugated fluorophores have been utilized (Kemmer et al., 2015; Veshaguri et al., 2016; Schwamborn et al., 2017; Gerdes et al., 2018; Kühnel et al., 2019). These lipid-conjugated pH sensors efficiently co-reconstitute with membrane proteins into large liposomes, thereby avoiding loss during reconstitution and allowing studies in reconstituted systems down to the single vesicle level (Kemmer et al., 2015; Veshaguri et al., 2016; Kühnel et al., 2019). Lipid-conjugated pH sensors were also instrumental to study the activity of lipases on native substrate systems (Bohr et al., 2020). Furthermore, short-chain lipid-conjugated pH sensors enable monitoring of pH changes from neutral to acidic conditions in the endocytic pathway of living cells (Kühnel et al., 2019).
Here, we describe the synthesis of lipid-conjugated pH sensors from amine-reactive pHrodo esters and phosphatidylethanolamines. The method described here enables fast preparation of the sensor and can be used as a template to couple other amine-reactive fluorophores to phosphatidylethanolamines.
Things to consider before starting
Choice of the fluorophore
The fluorophore must contain an amine-reactive functional group such as NHS- or STP-ester, to allow for coupling with the ethanolamine head group of the lipid. Furthermore, the fluorophore characteristics must be suitable for the later use of labelled lipids, e.g., pH range to induce a change in fluorescence emission. In Table 1, a number of reactive fluorophores are listed that we coupled effectively to phosphatidylethanolamines by using the protocol described here.
Choice of lipid
The lipid headgroup must contain an ethanolamine group to allow for the coupling reaction with amine-reactive dyes. The lipid chains can vary. Herein, dioleoyl- and dipalmitoylphosphatidylethanolamine (DOPE and DPPE, respectively) were employed. The usage of other lipids might lead to decreased yields, as the reaction conditions are optimized for DOPE and DPPE.
Choice of analysis method
A method to verify the successful formation of the labelled lipid is e.g., high resolution mass spectroscopy (MS), since small amounts of substance can be reliably detected. Two potential methods are MALDI-TOF MS (matrix-assisted laser desorption/ionization with time-of-flight analysis MS) and ESI MS (electrospray ionization MS).
Table 1. Fluorophores that have been coupled effectively to phosphatidylethanolamine (PE)Fluorophore Excitation (nm) Emission (nm) pH range# pKa# pHrodo Red NHS-ester 566 588 2.7–7.0 4.6 pHrodo Green STP-ester 505 543 2.7–7.0 4.6 SNARF-1 NHS ester 488–530 585/650† 6–11 9.3 Alexa FluorTM 488 NHS-ester 494 517 insensitive --- TAMRA NHS-ester 541 567 insensitive --- #Values upon coupling to DOPE (Kemmer et al., 2015; Kühnel et al., 2019).
†Values upon coupling to DOPE. Note that the emission spectrum of SNARF-1 undergoes a pH-dependent wavelength shift, thus allowing the ratio of the fluorescence intensities from the dye at two emission wavelengths to be used for more accurate determinations of pH.
All catalog numbers provided below shall serve as guide; alternative sources can be used as well.
Materials and reagents
All catalog numbers provided below shall serve as guide; alternative sources can be used as well.
Materials
Aluminium foil
Chromatography column, 200 × 10 mm, 15 mL (VWR, catalog number: 552-5410)
Disposable glass Pasteur pipettes (150 mm; VWR, catalog number: 612-1701)
Glass beads, 3 mm (Supelco, catalog number: 1040150500)
Glass cutter (Bohle, catalog number: BO 400.1)
Glass marbles (Fisher Scientific, catalog number: S04581)
Glass pipettes (e.g., graduated pipettes BLAUBRAND® Type 3 Class AS, 10 mL, graduation: 10 mL; Carl Roth, catalog number: HXT8.1)
Glass wool (VWR, catalog number: 519-3101)
Hamilton 700 Series Syringes of 25, 100, and 500 μL (Peter Oehmen GmbH, catalog numbers: 9221013, 9221015, 6055335)
Long necked glass tubes (Carl Roth, catalog number: 0486.2)
Magnetic bars ROTILABO® Micro, diameter 2 mm, length 5 mm (Carl Roth, catalog number: 0955.2)
Pipette tips 200 μL and 1,000 μL (SARSTEDT AG & Co. KG, catalog numbers: 70.760.002 and 70.3050.020)
Pointed-bottom glass tube, 10 mL (Merck, catalog number: CLS9950210)
Round-bottom glass tube, 10 mL, with joint and plug (Carl Roth, catalog number: NY90.1)
Spray bottle for primuline staining (Carl Roth, catalog number: YC44.1)
TLC plates, silica gel 60 (Merck, catalog number: 105626)
TLC chamber (Carl Roth, catalog number: 1N64.1)
Chemicals
Acetone p.a. (VWR, catalog number: 32201)
Ammonium molybdate tetrahydrate (Carl Roth, catalog number: 3666.1)
L-ascorbic acid (Carl Roth, catalog number: 3525.2)
Chloroform, ethanol-stabilized and certified for absence of phosgene and HCl (CHCl3) (Roth, catalog number: 7331.2)
CM SepharoseTM Fast Flowing (Sigma-Aldrich, catalog number: 17 0719-01)
Deionized water
Dichloromethane (DCM) (VWR, catalog number: 32222)
N’,N’-Diisopropylethylamine (DIPEA) (Sigma-Aldrich, catalog number: D125806)
N’,N’-Dimethylformamide (DMF) (Sigma-Aldrich, catalog number: D4551)
Ethanol p.a. (VWR, catalog number: 20821.321)
Methanol (MeOH) (VWR, catalog number: 20847.307)
Nitrogen gas, 99.999% (ALPHAGAZ, Air Liquide, Düsseldorf, Germany)
Perchloric acid (VWR, catalog number: 20589)
Primuline (Sigma-Aldrich, catalog number: 206865)
Di-sodium hydrogen phosphate dihydrate (VWR, catalog number: 28029)
Triethylamine (VWR, catalog number: 8.08352)
Ascorbic acid solution (see Recipes)
Extraction solution (see Recipes)
Molybdate solution (see Recipes)
Phosphate standard solution (see Recipes)
Primuline staining solution (see Recipes)
Reaction solvent (see Recipes)
TLC eluent (see Recipes)
Lipids
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE, Avanti Polar Lipids, Alabaster, AL, USA, catalog number: 850725)
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE, Avanti Polar Lipids, Alabaster, AL, USA, catalog number: 850705)
Note: This procedure has also been successfully performed using short-chain lipids including 1,2-dihexanoyl-sn-glycero-phosphoethanolamine (diC6-PE, catalog number: 850697), 1,2-dioctanoyl-sn-glycero-3-phosphoethanolamine (diC8-PE, catalog number: 850699), and 1-hexadecanoyl-2-hexanoyl-sn-glycero-3-phosphoethanolamine (C16C6-PE, custom made), all purchased from Avanti Polar Lipids (Alabaster, AL, USA).
Fluorescent dyes
pHrodo Red NHS-ester (Invitrogen, catalog number: P36600)
pHrodo Green STP-ester (Invitrogen, catalog number: P35369)
Alexa FluorTM 488 NHS-ester (Invitrogen, catalog number: A20000)
Note: This procedure has also been successfully performed using TAMRA NHS-ester (Lumiprobe, catalog number: 18120) and SNARF-1 NHS ester (Invitrogen, catalog number: S22801).
Equipment
Pipettes P200 and P1000 (GILSON®, catalog numbers: FD10005 and FD10006)
Analytical balance (Sartorius Entris-i II, 220 g/0.1 mg; Buch Holm, catalog number: 4669128)
Chemidoc MP imaging system (Bio-Rad) with illumination at 460–490 nm and 520–545 nm with 530/28 nm and 695/55 nm emission filters, respectively
CLARIOstar plate reader (BMG LABTECH)
Freezer -20 °C
Glass desiccator Boro 3.3 with socket in lid, 20 cm, including stopcock (BRAND GmbH, catalog number: 65238)
Heating block (Rotilabo® Block Heater H 250; Carl Roth, catalog number: Y264.1)
Magnetic stirrer (e.g., IKAMAG®, DREHZAHL ELECTRONIC, IKA, Staufen im Breisgau, Germany)
Refrigerated centrifuge (e.g., Multifuge® 3-R, Kendro Laboratory Products, catalog number: 75004371)
Vacuum Pump V-100 with interface I-100 and rotary evaporator Rotavapor® R-100, SJ29/32, V, 220–240V (Buchi, catalog numbers: 11593636, 11593655D, and 11100V111, 11061895)
Vortex mixer (Vortex Genie 2 Scientific Industries Inc., catalog number: SI-0236)
Water bath (Julabo CORIO C-BT5, catalog number: 9011305)
Software
ImageLab software version 5.2.1 (Bio-Rad)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Wiesner, W., Kühnel, R. M. and Pomorski, T. G. (2023). Synthesis and Purification of Lipid-conjugated Fluorescent pH Sensors. Bio-protocol 13(11): e4694. DOI: 10.21769/BioProtoc.4694.
分类
生物物理学 > 显微技术
药物发现 > 药物设计
生物化学 > 脂质 > 脂质测定
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













