- EN - English
- CN - 中文
In vivo Drug Screening to Identify Anti-metastatic Drugs in Twist1a-ERT2 Transgenic Zebrafish
在Twist1a-ERT2转基因斑马鱼中进行体内药物筛选以确定抗转移药物
发布: 2023年05月20日第13卷第10期 DOI: 10.21769/BioProtoc.4673 浏览次数: 2053
评审: Xi FengAmr Galal Abdelraheem IbrahimAlberto Rissone
Abstract
Here, we present an in vivo drug screening protocol using a zebrafish model of metastasis for the identification of anti-metastatic drugs. A tamoxifen-controllable Twist1a-ERT2 transgenic zebrafish line was established to serve as a platform for the identification. By crossing Twist1a-ERT2 with xmrk (a homolog of hyperactive form of the epidermal growth factor receptor) transgenic zebrafish, which develop hepatocellular carcinoma, approximately 80% of the double transgenic zebrafish show spontaneous cell dissemination of mCherry-labeled hepatocytes from the liver to the entire abdomen and tail regions in five days, through induction of epithelial to mesenchymal transition (EMT). This rapid and high-frequency induction of cell dissemination makes it possible to perform an in vivo drug screen for the identification of anti-metastatic drugs targeting metastatic dissemination of cancer cells. The protocol evaluates the suppressor effect of a test drug on metastasis in five days, by comparing the frequencies of the fish showing abdominal and distant dissemination patterns in the test drug–treated group with those in the vehicle-treated group. Our study previously identified that adrenosterone, an inhibitor for hydroxysteroid (11-beta) dehydrogenase 1 (HSD11β1), has a suppressor effect on cell dissemination in the model. Furthermore, we validated that a pharmacologic and genetic inhibition of HSD11β1 suppressed metastatic dissemination of highly metastatic human cell lines in a zebrafish xenotransplantation model. Taken together, this protocol opens new routes for the identification of anti-metastatic drugs.
Graphical overview
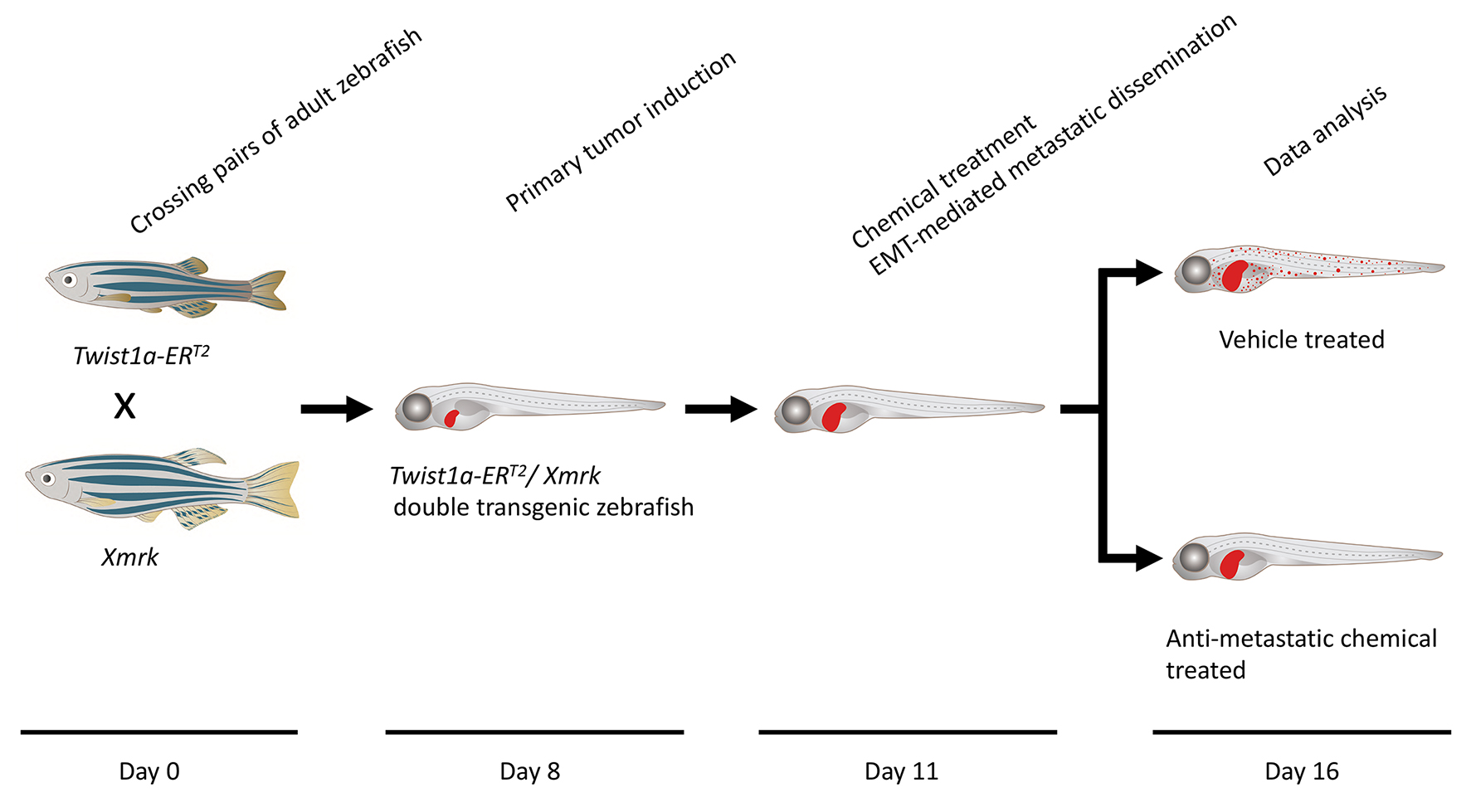
Timing
Day 0: Zebrafish spawning
Day 8: Primary tumor induction
Day 11: Chemical treatment
Day 11.5: Metastatic dissemination induction in the presence of a test chemical
Day 16: Data analysis
Keywords: In vivo drug screen (体内药物筛选)Background
Metastasis is responsible for approximately 90% of cancer-associated mortality. It proceeds through multiple steps: invasion, intravasation, survival in the circulatory system, extravasation, colonization, and metastatic tumor formation in secondary organs with angiogenesis (Nguyen et al., 2009; Chaffer and Weinberg, 2011; Welch and Hurst, 2019). The dissemination of cancer cells is an initial step of metastasis, and its molecular mechanism involves a local breakdown of basement membrane, loss of cell polarity, and induction of epithelial to mesenchymal transition (EMT). EMT plays a central role in early embryonic morphogenesis; its process enables various types of epithelial cells to convert into mesenchymal cells, through a downregulation of epithelial markers such as E-cadherin and an upregulation of mesenchymal markers such as vimentin. Twist, a basic helix-loop-helix transcription factor, plays a critical role in inducing the EMT program (Tsai and Yang, 2013; Lu and Kang, 2019). Past studies showed that elevated expression of Twist is associated with poor survival rates in patients with cancer; also, ectopic expression of Twist confers metastatic properties on cancer cells through induction of EMT (Yang et al., 2004; Tsai et al., 2012).
Cancer research using zebrafish as a model has attracted attention because this model offers many unique advantages that are not readily provided by other animal models (White et al., 2013; Osmani and Goetz, 2019). Furthermore, the zebrafish system has also been increasingly recognized as a chemical screening platform because it provides the advantage of high-throughput screening in an in vivo vertebrate setting with physiologic relevance to humans (Zon and Peterson, 2005; Letrado et al., 2018; Nakayama and Gong, 2020; Nakayama and Makinoshima, 2020; Nakayama et al., 2021b, 2022a and 2022b). Our study previously established a tamoxifen-controllable Twist1a-ERT2 transgenic zebrafish line that serves as an in vivo drug screening platform for the identification of anti-metastasis drugs targeting metastatic dissemination of cancer cells. By crossing Twist1a-ERT2 with xmrk (a homolog of hyperactive form of the epidermal growth factor receptor) transgenic zebrafish, which develop hepatocellular carcinoma, approximately 80% of the double transgenic zebrafish showed spontaneous cell dissemination of mCherry-labeled hepatocytes from the liver to the entire abdomen and tail regions in five days, through induction of an EMT (Nakayama et al., 2020; Lu et al., 2021). The dissemination patterns are generally divided into three categories: (i) local dissemination, in which disseminated mCherry-positive cells exist in close proximity to the liver; (ii) abdominal dissemination, in which the cells spread throughout the abdomen; and (iii) distant dissemination, in which the cells are observed over a broad region from the trunk to the tail (Figure 1A).
This rapid and high-frequency induction of cell dissemination makes it possible to perform an in vivo drug screen for the discovery of anti-metastasis drugs targeting metastatic dissemination of cancer cells. The protocol evaluates the suppressor effect of a test chemical through comparing the frequencies of the fish showing the abdominal and distant dissemination patterns in the test drug–treated group with those in the vehicle-treated group. Previous studies confirmed that ki16425 (a LPA1 inhibitor) or Y27632 (an inhibitor of Rho-associated coiled-coil-containing protein kinase), which have been reported to suppress metastasis in mice models of metastasis (Itoh et al., 1999; Boucharaba et al., 2006), could suppress cell dissemination in the fish model. In vivo drug screen using this model identified adrenosterone, an inhibitor for hydroxysteroid (11-beta) dehydrogenase 1 (HSD11β1), as having a potential to suppress metastatic dissemination of cancer cells (Figure 1B and 1C). Furthermore, pharmacologic and genetic inhibition of HSD11β1 were validated to suppress metastatic dissemination of highly metastatic human cell lines in a zebrafish xenotransplantation model (Nakayama et al., 2020 and 2021a). Taken together, our model offers an in vivo drug screening platform for the identification of anti-metastatic drugs.
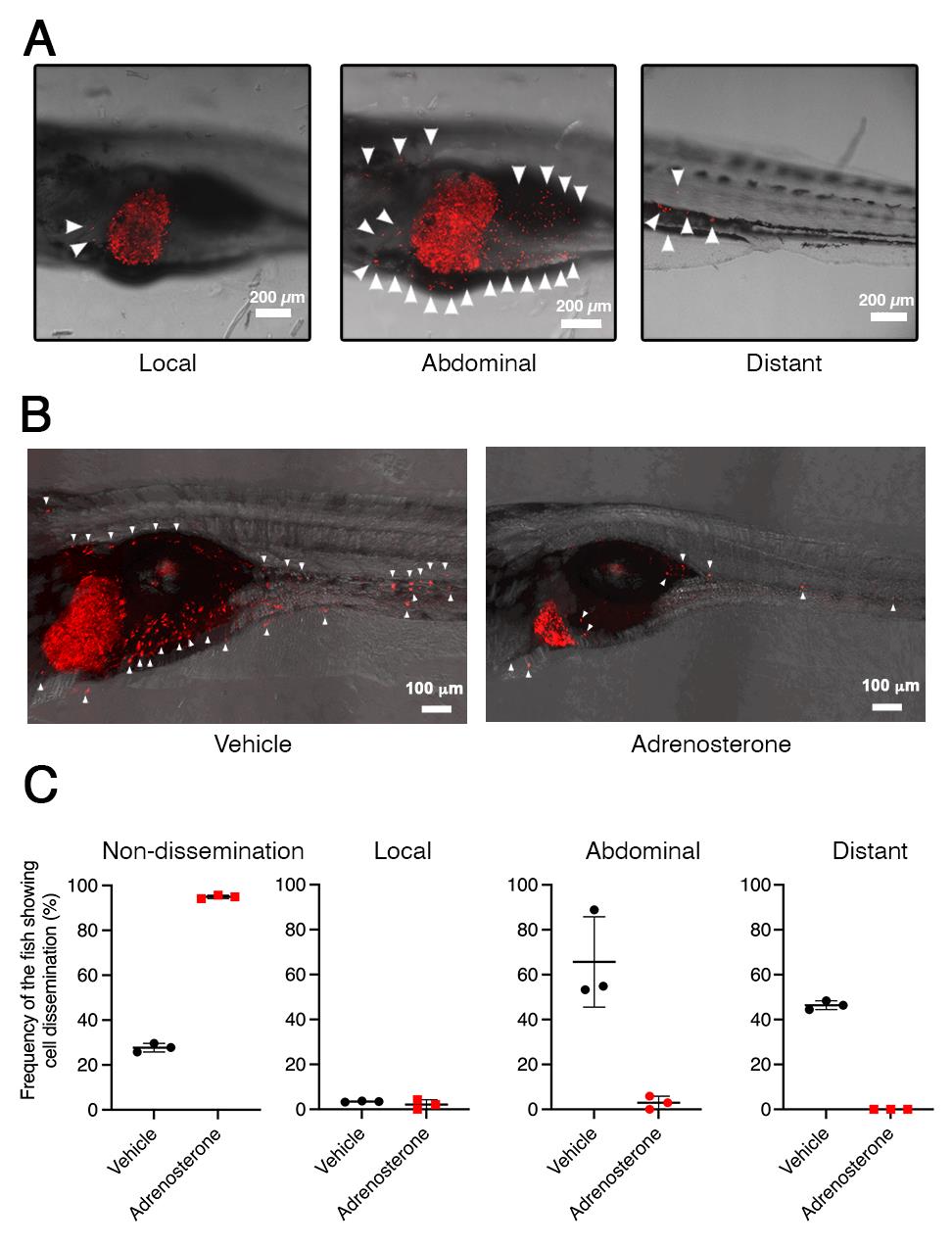
Figure 1. Twist1a-ERT2/xmrk double transgenic zebrafish offers an in vivo drug screening platform for the identification of anti-metastatic drugs. A. Representative images of the dissemination of mCherry-labeled hepatic cells from the liver in Twist1a-ERT2/xmrk double transgenic zebrafish at 16 days post-fertilization (dpf). The fish were treated with doxycycline and 4-hydroxytamoxifen (4-OHT). Some of the disseminated mCherry-positive cells are indicated by arrowheads. The images are shown as Z-stack images using 100× magnification. Scale bar, 200 μm. B. Representative images of the effect of adrenosterone on the dissemination of mCherry-positive cells in the fish at 16 dpf. Fish were treated with either vehicle (left) or adrenosterone (right). The images are shown as Z-stack images using 100× magnification. Scale bar, 100 μm. C. Mean frequencies of the fish showing the dissemination patterns of mCherry-positive cells in the vehicle- or adrenosterone-treated groups. Each value is presented as mean ± SEM from three independent experiments. Images are reprinted from Nakayama et al. (2020).
Materials and Reagents
150 mm dish (Corning, catalog number: 430599)
100 mm dish (Corning, catalog number: 430167)
6-well flat-bottom plastic plates (Corning, catalog number: CLS3335)
Micron powder food (food for zebrafish larvae) (Sera)
27 G needle tip (Terumo, catalog number: NN-2719S)
Plastic tea strainer (purchased from a local supermarket)
Transgenic zebrafish line Tg (fabp10a:mCherry-T2A-Twist1a-ERT2)
Transgenic zebrafish line Tg (fabp10a:TA; TRE:xmrk; krt4:GFP) known as xmrk
Note: Tg (fabp10a:mCherry-T2A-Twist1a-ERT2) and Tg (fabp10a:TA; TRE:xmrk; krt4:GFP) are available upon request from Prof. Zhiyuan Gong, Department of Biological Sciences, National University of Singapore, Singapore.
Doxycycline (Sigma-Aldrich, catalog number: D9891)
4-hydroxytamoxifen (4-OHT) (Sigma-Aldrich, catalog number: H6278)
Methylcellulose (Sigma-Aldrich, catalog number: M7027)
Phenoxyethanol (Sigma-Aldrich, catalog number: 77699)
NaCl (Sigma-Aldrich, catalog number: S3014)
KCl (Sigma-Aldrich, catalog number: P9541)
MgSO4·7H2O (Sigma-Aldrich, catalog number: M2773)
CaCl2 (Sigma-Aldrich, catalog number: C4901)
Doxycycline stock solution (see Recipes)
4-hydroxytamoxifen stock solution (see Recipes)
E3 medium (see Recipes)
30% methylcellulose (w/v %) (see Recipes)
Phenoxyethanol (v/v %) (see Recipes)
Equipment
Recirculating aquaculture system (Aquatic Habitat)
Fluorescence microscope (Olympus, catalog number: MVX10)
Leica TCS SP5X confocal microscope system (Leica)
Incubator for zebrafish embryos (AQUALYTIC, catalog number: 2418210)
External tank, a component of zebrafish breeding tank (Tecniplast, catalog number: ZB10BTE)
Perforated internal tank, a component of zebrafish breeding tank (Tecniplast, catalog number: ZB10BTI)
Polycarbonate divider, a component of zebrafish breeding tank (Tecniplast, catalog number: ZB10BTD)
Polycarbonate lid (Tecniplast, catalog number: ZB10BTL)
Software
Image analysis software, Imaris 8 (Bitplane)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Nakayama, J., Makinoshima, H. and Gong, Z. (2023). In vivo Drug Screening to Identify Anti-metastatic Drugs in Twist1a-ERT2 Transgenic Zebrafish. Bio-protocol 13(10): e4673. DOI: 10.21769/BioProtoc.4673.
分类
药物发现 > 药物筛选
癌症生物学 > 侵袭和转移 > 药物发现和分析 > 细胞迁移
细胞生物学 > 细胞运动 > 细胞运动性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













