- EN - English
- CN - 中文
Novel Antibody-independent Method to Measure Complement Deposition on Bacteria
测量细菌补体沉积的独立于抗体的新方法
发布: 2023年05月05日第13卷第9期 DOI: 10.21769/BioProtoc.4671 浏览次数: 1482
评审: Alessandro DidonnaAnonymous reviewer(s)
Abstract
During infection, complement plays a critical role in inflammation, opsonisation, and destruction of microorganisms. This presents a challenge for pathogens such as Staphylococcus aureus to overcome when invading the host. Our current knowledge on the mechanisms that evolved to counteract and disable this system is limited by the molecular tools available. Present techniques utilise labelled complement-specific antibodies to detect deposition upon the bacterial surface, a method not compatible with pathogens such as S. aureus, which are equipped with immunoglobulin-binding proteins, Protein A and Sbi. This protocol uses a novel antibody-independent probe, derived from the C3 binding domain of staphylococcal protein Sbi, in combination with flow cytometry, to quantify complement deposition. Sbi-IV is biotinylated, and deposition is quantified with fluorophore-labelled streptavidin. This novel method allows observation of wild-type cells without the need to disrupt key immune modulating proteins, presenting the opportunity to analyse the complement evasion mechanism used by clinical isolates. Here, we describe a step-by-step protocol for the expression and purification of Sbi-IV protein, quantification and biotinylation of the probe, and finally, optimisation of flow cytometry to detect complement deposition using normal human serum (NHS) and both Lactococcus lactis and S. aureus.
Keywords: Complement deposition (补体沉积)Background
Staphylococcus aureus has become a major global burden due to widespread infection within hospitals and communities (Murray et al., 2022). Due to the prevalence of antibiotic resistance, chronic-infections, and surgical complications, the demand to understand this pathogen and its relationship with the host has become critical (Tong et al., 2015). For a successful infection, pathogens must combat the first line of innate defence, the complement system. Complement is quick to identify pathogens, label them for efficient phagocytosis, and raise the alarm to other immune effectors of both the innate and adaptive systems (Noris and Remuzzi, 2013). For successful infection, pathogens must disable this pathway. S. aureus has a surprisingly large arsenal of complement-disabling proteins; however, due to restrictions in phenotypic assays, the details of virulence factors involved are not fully understood (Thammavongsa et al., 2015). The common method for observing complement activity/deposition utilises labelled antibodies that are detected by methods such as flow cytometry. In the case of S. aureus, this method is not possible due to the expression of two immunoglobulin binding proteins, Protein A (Spa) and Sbi, which bind indiscriminately to the Fc region of antibodies (Yang et al., 2018; Cruz et al., 2021). Previous attempts to bypass this issue used mutants unable to express IgG binding proteins (∆spa∆sbi); however, both proteins are important virulence factors in the defence against complement activation and are known to be expressed in >95% of clinical isolates.
In our study, we designed a novel probe utilising the C3 binding domain of Sbi (domain IV, see Figure 1) and biotinylated it to allow recognition by fluorophore-labelled streptavidin (Clark et al., 2011; Wonfor et al., 2022). We show that it is able to bypass Spa/Sbi interference, and binding is specific to C3 deposition. This probe opens the door to analysing the immune evasion capacity of large collections of clinical isolates, allowing for a greater understanding of complement evasion at a population level. Staphylococci are not the only pathogens to bind IgG: Streptococcus has protein G, and Peptostreptococcus has protein L; therefore, this method may also enlighten the role of complement in other pathogen invasions (Sidorin and Solov'eva, 2011). Finally, Sbi-IV probe has the advantage of being very small in size. As a 11 kDa protein, in comparison to 150 kDa IgG antibody, the probe may have advantages outside immunoglobulin binding pathogens, and we envisage a role in in vivo diagnostics, such as detecting tissue inflammation.
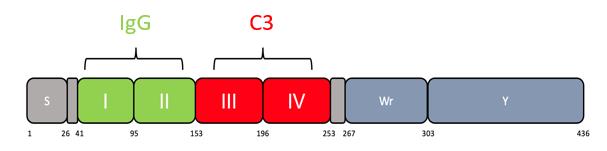
Figure 1. Scheme showing domains within Sbi protein. Residue numbers are shown below. Domains I and II bind IgG. Domains III and IV bind C3. Domain IV consists of residues 196–253. Additional residues added ensure stability and correct folding (Smith et al., 2012).
Materials and Reagents
General lab reagents
Beakers, 250 mL
Eppendorf: 1.5 mL, 2 mL
Falcons: 15 mL, 50 mL
Flasks 2 L
Flea (magnetic stirring bar)
Foil
Petri dish/staining box
Syringe: 5 mL, 10 mL, 50 mL
Syringe needles
Centrifuge bottles compatible with specific floor centrifuge rotor (1 L, 50 mL)
Filter 0.45 μm (Thermo Fisher, Millipore, catalog number: SLHA033SS)
His-Trap column 1 mL (Fisher Scientific, Cytiva, catalog number: 10431065)
Microcuvettes 1.6 mL (Fisherbrand, catalog number: FB55147)
Size Exclusion Column HiLoad 16/600 Superdex 200 prep grade, 120 mL (Fisher Scientific, Cytivia, catalog number: 45002490)
Round bottom 96-well plate (Corning, catalog number: 3788)
Transfer pipette 5 mL sterile (Sigma, catalog number: HS206371C)
TurboBlot transfer pack (nitrocellulose membrane 0.2 μm) (Bio-Rad, catalog number: 1704158)
V-bottomed 96-well plate (Thermo Fisher, Nunc, catalog number: 249570)
BamHI-HF (NEB, catalog number: R3136S)
BL21 DE3 competent cells (NEB, catalog number: C2527I). Alternatively, make your own competent cells using CaCl2
GeneJET Miniprep kit (Thermo Fisher, catalog number: K0502)
HindIII-HF (NEB, catalog number: R3104S)
NovaBlue competent cells (Sigma, catalog number: 70181-3)
Phusion High-Fidelity PCR master mix (Thermo Fisher, catalog number: F351S)
T4 DNA ligase (NEB, catalog number: M0202S)
Wizard SV Gel and PCR clean up kit (Promega, catalog number: A9281)
Ampicillin (Fisher Scientific, catalog number: BP176025). Dilute powder in ddH2O to a final concentration of 100 mg/mL stock (store dilution at -20 °C)
Coomassie Brilliant Blue R-250 destain (Bio-Rad, catalog number: 1610438)
Coomassie Brilliant Blue R-250 stain (Bio-Rad, catalog number: 1610436)
Ethanol absolute (VWR, catalog number: 20821.330). Dilute to 20% with water
IPTG (Sigma, catalog number: 16758). Dilute powder in ddH2O to a final concentration of 0.5 M (store dilution at -20 °C)
Imidazole (Sigma, catalog number: I2399)
LB broth (Sigma, catalog number: L3022)
Laemmli sample buffer 4× (Bio-Rad, catalog number: 1610747)
mPAGE SDS running buffer powder (Sigma, catalog number: 20347927)
NaCl (Sigma, catalog number: S7653)
PageRuler Plus Prestained protein ladder (Thermo Fisher, catalog number: 26619)
Protease Inhibitor Cocktail Set VII (Sigma, Millipore Corp, catalog number: 539138)
SDS-PAGE precast gel 12% (Bio-Rad, catalog number: 4561043)
Trizma HCl (Sigma, catalog number: T3253)
Vivaspin 20 (5 kDa MWCO) (Sigma, catalog number: Z614580)
Vivaspin 500 (5 kDa MWCO) (Sigma, catalog number: Z614009)
BCA Protein Assay kit (Thermo Fisher, Pierce, catalog number: 23225)
BD Vacutainer® Clot Activator Tube (BD, catalog number: 367895)
ECL detection reagent (Cytivia, Amersham, catalog number: RPN2235)
Mini-PROTEAN TGX precast gel 4%–20% 10 wells (Bio-Rad, catalog number: 4561094)
Phosphate buffered saline (PBS) tablets (Thermo Fisher, Oxoid, catalog number: BR0014G)
Expression plasmid pQE30-Sbi-IV, available upon request
EZ-LinkTM Sulfo-NHS-Biotinylation kit (Thermo Fisher, catalog number: 21425)
Skim milk powder (BD, Difco catalog number: 232100)
TBST powder (Sigma, catalog number: T9039)
Ultra-streptavidin-HRP (Thermo Scientific, catalog number: N504)
Barbituric acid (Sigma, catalog number: 185698)
Bovine serum albumin (BSA) (Sigma, Roche, catalog number: 03117332001)
Calcium chloride (CaCl2) (Sigma, catalog number: 223506)
Cell trace Far Red (Thermo Fisher, Invitrogen, catalog number: C34564)
FC loading tubes (5 mL polystyrene round-bottom test tube) (Fisher Scientific, Falcon, catalog number: 352052)
Fetal calf serum (FCS) (Thermo Fisher, Gibco, catalog number: A5256701)
Gelatin (from porcine skin) (Sigma, catalog number: G1890)
Compstatin (AMY-101) (MedChemExpress, catalog number HY-P1717)
Glucose (Sigma, catalog number: G8270)
Glass universal (for growing S. aureus) (Fisher Scientific, catalog number: 14823562)
M17 broth (Sigma, catalog number: 56156)
Magnesium chloride (MgCl2) (Sigma, catalog number: M8266)
Na-barbital (sodium 5,5-diethylbarbiturate) (Sigma, catalog number: B0500)
Streptavdidin-488 (Thermo Fisher, Invitrogen, catalog number: S32354)
Tryptic soy broth (Sigma, catalog number: T8907)
His A buffer (see Recipes)
His B buffer (see Recipes)
SEC buffer (see Recipes)
Milk block (see Recipes)
M17-G broth (see Recipes)
10% FCS/PBS (see Recipes)
BSA/PBS (see Recipes)
Veronal buffer stock (VBS) (25 mM) (see Recipes)
GVB++ buffer (see Recipes)
Equipment
Forceps (Fisher Scientific, catalog number: 15281209)
AKTA purifier 10 (Amersham Pharmacia Biotech)
Belly dancer/rocker (Fisherbrand, 3D Platform Rotator)
Bench centrifuge with compatible rotor for Eppendorfs capable of reaching speeds of 16,000× g
Centrifuge capable of speeds of at least 60,000× g and compatible with rotors for 50 mL and 1 L bottles (e.g., Beckman Avanti)
Centrifuge with buckets compatible for 96-well plates capable of reaching 3,400× g (Eppendorf, model: 5810 R)
Electrophoresis Power Supply (Cleaver Scientific, model: PowerPRO 300)
FACS CANTO Flow Cytometer (BD)
Falcon centrifuge capable of reaching 3,400× g (Eppendorf, model: 5810 R)
Freezer (-80 °C) for storage of serum and Sbi-IV protein
Gel imaging system (Azure Biosystem 400) for viewing chemiluminescence
Heat block reaching temperatures of 95 and 56 °C (Fisherbrand, Isotemp)
Magnetic stirrer (Stuart, model: US152)
NanoDrop/spectrophotometer (absorbance at 600 nm) (DeNovix, model: DS-11)
Protein electrophoresis tank (Bio-Rad, model: Mini-PROTEAN Tetra Cell)
Shaking incubator (capable of holding 2 L flasks and shaking at 200 rpm) set to 37 °C (e.g., Stuart SI500)
Sonicator (Soniprep 150 plus)
Spectrophotometer (absorbance at 562 nm) (Tecan, Sunrise)
Static incubator, set to 37 °C (Fisher Scientific, HERATherm, catalog number: 10744262)
Thermocycler (Applied Biosystem, SimpliAmp)
Trans-Blot Turbo Transfer System (Bio-Rad)
Western Blot Roller (Thermo Fisher, catalog number: 84747)
Software
BD FACSDiva (BD) for flow cytometer
FlowJoTM v10 software (BD Life Sciences) (www.flowjo.com)
GraphPad Prism 8 (Dotmatics) (www.graphpad.com)
Unicorn 5.32 (Cytiva) for AKTA
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Wonfor, T., Li, S. and Laabei, M. (2023). Novel Antibody-independent Method to Measure Complement Deposition on Bacteria. Bio-protocol 13(9): e4671. DOI: 10.21769/BioProtoc.4671.
分类
微生物学 > 微生物-宿主相互作用 > 细菌
免疫学 > 补体分析
分子生物学 > 蛋白质 > 流式细胞术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link