- EN - English
- CN - 中文
Murine Double Hit Model for Neonatal Cardiopulmonary Diseases: Bronchopulmonary Dysplasia (BPD) and Pulmonary Hypertension Associated with BPD
新生儿心肺疾病的小鼠双击模型:支气管肺发育不良(BPD)和与BPD相关的肺动脉高压
发布: 2022年11月05日第12卷第21期 DOI: 10.21769/BioProtoc.4669 浏览次数: 4400
评审: Vivien Jane Coulson-ThomasJaira Ferreira de VasconcellosAlberto Rissone
Abstract
Bronchopulmonary dysplasia (BPD) and pulmonary hypertension associated with BPD (BPD-PH) are of multifactorial origin and share common risk factors. Most murine models of BPD expose newborn pups to only one of these risk factors—more commonly postnatal hyperoxia—thereby mimicking the vital increased fraction of inspired oxygen (FiO2) that preterm infants in neonatal intensive care units often require. To improve representation of the multifactorial origins of BPD and BPD-PH, we established a double hit model, combining antenatal systemic inflammation followed by postnatal hyperoxia. On embryonic day 14, pups are exposed to systemic maternal inflammation via a single intraperitoneal injection of 150 µg/kg of lipopolysaccharide to the dam. Within 24 h after birth, pups and dams are randomized and exposed to gas with either an FiO2 of 0.21 (room air) or 0.65 (hyperoxia 65%). In our BPD and BPD-PH double hit model, we can obtain multiple readouts from individual pups that include echocardiography, lung histology and immunohistochemistry, ex vivo X-ray micro computed tomography, and pulmonary and plasmatic immunity by RNA, protein, or flow cytometry.
Graphical abstract:
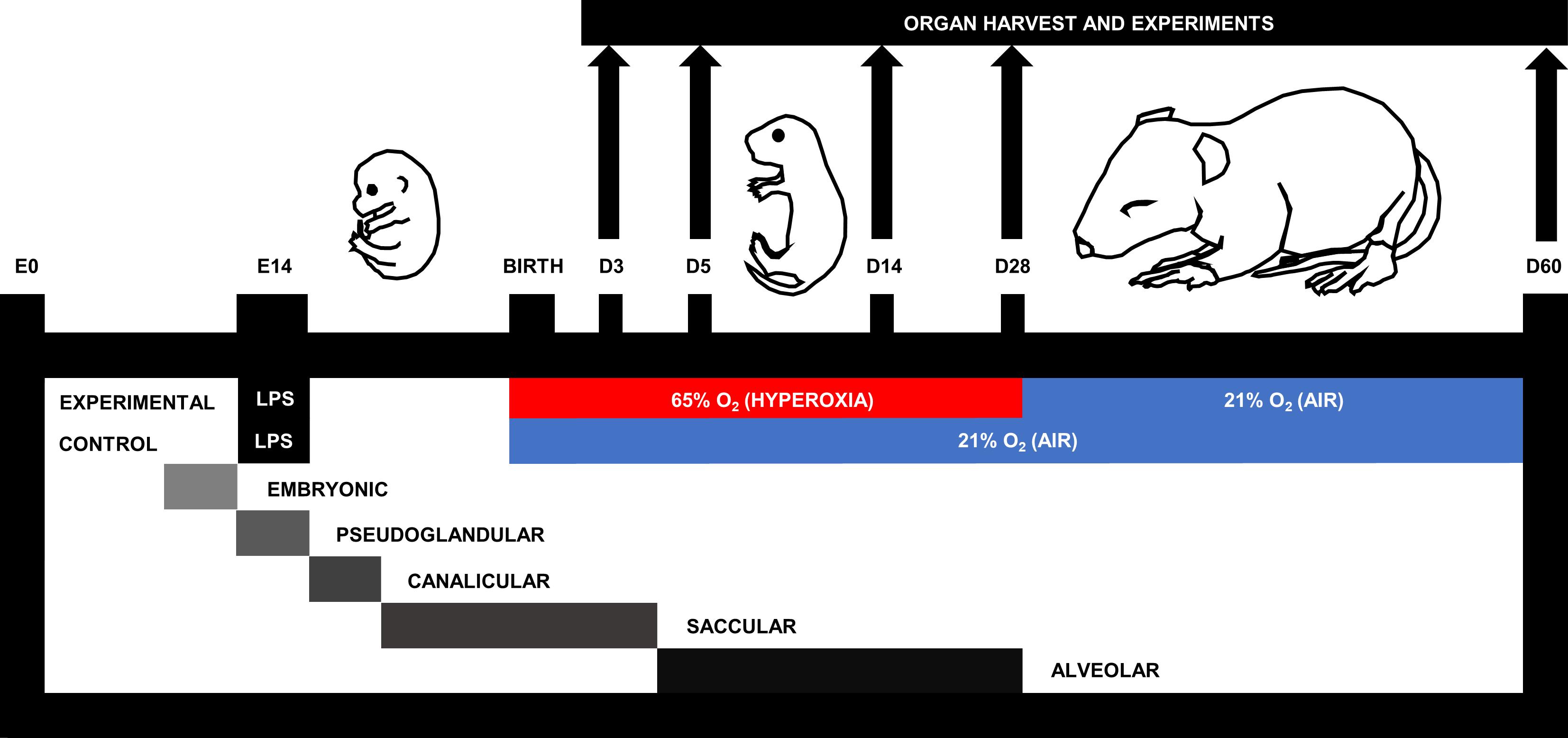
Figure 1. Murine double hit model of cardiopulmonary disease. On embryonic day (E)14, pups are exposed to systemic maternal inflammation via a single intraperitoneal injection of 150 µg/kg lipopolysaccharide to the dam. Within 24 h after birth, pups and dams are randomized to be exposed to gas with either a fraction of inspired oxygen (FiO2) of 0.21 (air; 21% O2) or 0.65 (hyperoxia; 65% O2) for a maximum of 28 days. According to the murine stage of lung development (Schittny, 2017), experimental endpoints include postnatal day (D)3, D5, D14, D28, and D60.
Background
Bronchopulmonary dysplasia (BPD) and pulmonary hypertension secondary to BPD (BPD-PH) are severe cardiopulmonary morbidities of premature infants that are underpinned by a surge in pulmonary inflammation. Pulmonary inflammation interrupts alveolar and vascular development, compromising gas exchange in infants with BPD (Thebaud et al., 2019). According to a systematic review and meta-analysis, approximately 17% of all infants with BPD develop BPD-PH, with a mortality rate of 14%–38% (Al-Ghanem et al., 2017). BPD-PH occurs due to a reduced cross-sectional area of the pulmonary vasculature that increases pulmonary vascular resistance and hence blood pressure (Parker and Abman, 2003; Khemani et al., 2007).
Pulmonary inflammation in the preterm infant can originate from many sources, with maternal inflammation being particularly prominent (Kemp, 2014). Environmental factors (stress, alcohol, smoking), autoimmune conditions (lupus erythematosus, inflammatory bowel disease, diabetes), and inflammatory pregnancy conditions (preeclampsia, chorioamnionitis) all contribute to a pro-inflammatory uterine environment in the mother. Infants with chorioamnionitis present with increased umbilical serum levels of pro-inflammatory cytokines including IL-1β, IL-6, and TNF (Dollner et al., 2002), which are important malefactors in BPD and BPD-PH (Sahni et al., 2020). Systemic maternal inflammation can be simulated in mice with an intraperitoneal (i.p.) injection of lipopolysaccharide (LPS), a bacterial endotoxin that binds to the toll-like receptor 4, a pattern recognition receptor of the innate immune system that promotes expression of pro-inflammatory cytokines, including IL-1β, TNF, and IL-6 (Palsson-McDermott and O'Neill, 2004). In pregnant rats, i.p. injection of 2.5 mg/kg LPS at gestational day 20 and 21 (full term = 22 days) resulted in prolonged pulmonary inflammation and delayed lung maturation in the offspring (Cao et al., 2009).
Unfortunately, early life inflammation is perpetuated postnatally by lifesaving interventions received in the neonatal intensive care unit. The most prevalent is the use of an increased fraction of inspired oxygen (FiO2) (hyperoxia) delivered to the preterm infant to counteract the reduced diffusing capacity of the immature lung. Although hyperoxia saves the life of the infant, it also comes with the unfortunate consequence of increased pulmonary inflammation via the production of reactive oxygen species (Torres-Cuevas et al., 2017).
Mimicking multifactorial diseases by including multiple pathophysiological drivers into in vivo models will lead to greater methodological rigor for faster translation of lifesaving therapies into clinics. However, to date, many murine BPD models only use one insult, namely hyperoxia. At the time we introduced our model, to the best of our knowledge, double hit strategies had been applied only twice to mimic BPD in newborn rodents (Choi et al., 2009; Velten et al., 2010), whereas there was none established to assess BPD-PH. Choi et al. (2009) first recognized the relevance of a double hit model, discovering that intra-amniotic LPS (0.5 or 1.0 mg) administration at day 20 of gestation (full term = 22.5) in neonatal rats amplified the lung injury induced by one week of exposure to hyperoxia (85% O2). One year later, Velten et al. (2010) injected 80 µg/kg LPS (i.p.) to pregnant mice at day 16 of gestation (full term = 19-21); upon birth, they placed pups in hyperoxia (85% O2) for 14 days followed by room air for another 14 days. These pups presented with a phenotype of arrested alveolarization, diffuse fibrosis, and impaired lung mechanics.
Expanding on these BPD models, we set out to establish a murine double hit model of early life cardiopulmonary disease representative for BPD and BPD-PH (Nold et al., 2013) (Figure 1). First, dams are injected (i.p.) with 150 µg/kg of lipopolysaccharide on day 14 of gestation, and upon birth pups are subsequently placed in hyperoxia (65% O2) or housed under room air control conditions. Pups subjected to this double hit develop alveolar and vascular changes representative of clinical features in BPD and BPD-PH. Our double hit model distinguishes itself from the earlier combined models by minimizing the FiO2 (65% compared to 85%) to better reflect clinical practice. Intuitively, we hypothesized that any improvement in BPD would most likely also improve BPD-PH, because of the inextricable link between alveolarization and angiogenesis.
Our team has established an experimental endpoint protocol so we could simultaneously investigate combinations of multiple individual readouts from every individual pup, including ex vivo (lung histology, immunohistochemistry, flow cytometry, x-ray micro computed tomography, precision cut lung slices, pulmonary/plasmatic immunity) and in vivo readouts (cine-angiography, echocardiography). Plethysmography, behavioral changes, and long-term neurodevelopmental outcomes could be considered as additional in vivo outcomes.
Our discovery that neonatal cardiopulmonary disease is underpinned by type 2 immunity (Lao et al., 2022) confirmed a pathogenetic similarity between BPD and asthma that has been speculated (de Kleer et al., 2016) but never fully proven. Proof of concept from our murine BPD/PH model (Figure 1) in a STAT6 deficient mouse, which lacks a full type 2 immune response, confirmed that the type 2 pathway is critical to the pathophysiology of BPD/PH and that blockade of type 2 pathways is highly effective in preventing the disease. This revelation might shift the current paradigm for treating BPD, which relies on gentle ventilation techniques and, ultimately, the use of corticosteroids as a rescue therapy. In the future, seeing BPD and BPD-PH as a type 2 disease will open up the use of well tolerated anti-type 2 drugs as a novel treatment strategy. In addition, our preclinical studies, in which we apply our model and other early life disease models, have shown that early and effectively blocking inflammation, specifically the potent pro-inflammatory interleukin (IL)-1, also holds great promise for preventing BPD and BPD-PH (Nold et al., 2013; Royce et al., 2016; Bui et al., 2019). Blocking IL-1 using its natural adversary IL-1 receptor antagonist (IL-1Ra, drug name anakinra) has an excellent safety and efficacy record, which has been established over two decades via anakinra’s clinical use in adults, children, and infants, as treatment for neonatal onset multi-system inflammatory disease (Sibley et al., 2012).
Materials and Reagents
Ultra-fine 31G insulin syringes 0.5 mL (Becton Dickinson, catalog number: 32281)
InsyteTM AutoguardTM winged, 24G × 0.75” (Becton Dickinson, catalog number: 381212)
MicrolanceTM hypodermic needle 30G × 0.5 (Becton Dickinson, catalog number: 304000)
10 µL gastight syringe, cemented needle, 26s gauge, 2 in., point style 2 (Hamilton, catalog number: 1701)
C57BL/6J mice
Lipopolysaccharide (LPS) from Escherichia coli O127:B8; purified by phenol extraction (Sigma, catalog number: L3129)
Sodium chloride intravenous infusion BP (water for injection) (Pfizer, catalog number: AUST R 49280)
Oxygen gas
Isoflurane (AbbVie, Forthane®, catalog number: AUST R 29656, store below 30 °C)
Paraformaldehyde (PFA) powder, 95% (Sigma-Aldrich, catalog number: 158127)
PBS (ThermoFisher Scientific, GibcoTM, catalog number: 14190250)
Cotton tip applicators (BSN Medical, catalog number: 7505-0)
Gauze
Liquid nitrogen (N2)
Wet ice
Polyester thread (sutures) (Birch, catalog number: 004601)
1.7 mL microtubules (Axygen, catalogue number: MCT-175-C)
Equipment
Custom plexiglass gas chamber [approximate dimensions: 40 × 125 × 30 (L × W × H) cm]
Bear cub BP2001 infant ventilator (Bear Medical Systems, Type #240)
Portable oxygen analyzer (Teledyne, model: AX300-I)
Oxygen sensor (Maxtec, model: R109P09)
Power lab/16SP (ADinstruments, model: ML795)
Heated respiratory humidifiers (Fisher and Paykel, model: MR730)
Autofill humification chamber (Fisher and Paykel, model: MR290)
Temperature pod (ADinstruments, model: ML312)
Pod expander (ADinstruments, model: ML305)
Compact scale, 510 g × 0.1 g (A&D Weighing, model: HT-500)
Gravity perfusion apparatus
Forceps, tip width 0.5 mm, length 10 cm (Fine Science Tools, catalog number: 11150-10)
Scissors (Precision Medical Specialties, model: E25-500)
Foam dissection tray
Liquid nitrogen container (Nalgene®, catalog number: 4150-2000)
-86 °C freezer 690 L (Froilabo, model: BMEVO69086G)
Software
Chart 5 Pro v5.5.1 (ADInstruments, www.ADInstruments.com)
Procedure
文章信息
版权信息
© 2022 The Authors; exclusive licensee Bio-protocol LLC.
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Garrick, S. P., Berger, P. J., Nold, M. F. and Nold-Petry, C. A. (2022). Murine Double Hit Model for Neonatal Cardiopulmonary Diseases: Bronchopulmonary Dysplasia (BPD) and Pulmonary Hypertension Associated with BPD. Bio-protocol 12(21): e4669. DOI: 10.21769/BioProtoc.4669.
- Lao, J. C., Bui, C. B., Pang, M. A., Cho, S. X., Rudloff, I., Elgass, K., Schroder, J., Maksimenko, A., Mangan, N. E., Starkey, M. R., et al. (2022). Type 2 immune polarization is associated with cardiopulmonary disease in preterm infants. Sci Transl Med 14(639): eaaz8454.
分类
免疫学 > 动物模型 > 小鼠
医学 > 心血管疾病
细胞生物学 > 组织分析 > 损伤模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link