- EN - English
- CN - 中文
Simultaneous Microendoscopic Calcium Imaging and EEG Recording of Mouse Brain during Sleep
小鼠睡眠期间大脑的同时显微内窥镜钙成像和脑电图记录
发布: 2023年05月05日第13卷第9期 DOI: 10.21769/BioProtoc.4664 浏览次数: 2498
评审: Geoffrey C. Y. LauShengjin XuBo Liang
Abstract
Sleep is a conserved biological process in the animal kingdom. Understanding the neural mechanisms underlying sleep state transitions is a fundamental goal of neurobiology, important for the development of new treatments for insomnia and other sleep-related disorders. Yet, brain circuits controlling this process remain poorly understood. A key technique in sleep research is to monitor in vivo neuronal activity in sleep-related brain regions across different sleep states. These sleep-related regions are usually located deeply in the brain. Here, we describe technical details and protocols for in vivo calcium imaging in the brainstem of sleeping mice. In this system, sleep-related neuronal activity in the ventrolateral medulla (VLM) is measured using simultaneous microendoscopic calcium imaging and electroencephalogram (EEG) recording. By aligning calcium and EEG signals, we demonstrate that VLM glutamatergic neurons display increased activity during the transition from wakefulness to non-rapid eye movement (NREM) sleep. The protocol described here can be applied to study neuronal activity in other deep brain regions involved in REM or NREM sleep.
Keywords: Sleep (睡眠)Background
Sleep and wakefulness are actively controlled by the interplay of distinct neural circuits in the brain (Weber and Dan, 2016; Scammell et al., 2017). Centered on the mutual inhibition between sleep- and wakefulness-promoting circuits, a flip-flop model has been proposed to explain the mechanisms of sleep-wake state transitions (Saper et al., 2001; Saper et al., 2010). The discovery of the ascending reticular activating system (Moruzzi and Magoun, 1949), the orexin neurons (de Lecea et al., 1998; Sakurai et al., 1998), and further studies of neuromodulatory systems have greatly advanced our understanding of the neural circuits supporting wakefulness (Brown et al., 2012; Lee and Dan, 2012; Scammell et al., 2017). By contrast, the neural circuits controlling sleep have remained elusive. Several sleep-promoting regions have been identified, including the preoptic area (POA, particularly the ventrolateral preoptic area and median preoptic nucleus, or VLPO and MPO, respectively) (Sherin et al., 1996; John and Kumar, 1998; Lu et al., 2000; Alam et al., 2014; Kroeger et al., 2018), the parafacial zone (Anaclet et al., 2012 and 2014), and the basal forebrain (Xu et al., 2015). However, whether these brain structures are involved in sleep initiation or maintenance remains largely unknown. GABAergic neurons in these brain regions are sleep-active, and their activation promotes non-rapid eye movement (NREM) sleep (Scammell et al., 2017). In addition to the role of GABAergic neurons in sleep regulation, recent studies have identified glutamatergic neurons that promote NREM sleep in a few brain areas, including the perioculomotor region of the midbrain (Zhang et al., 2019), the ventrolateral periaqueductal gray of the midbrain (Zhong et al., 2019), and the posterior thalamus (Ma et al., 2019).
The POA is the most intensively studied sleep-active and sleep-promoting region. Immunohistochemistry studies in rats showed the existence of sleep-active GABAergic neurons in the VLPO and a positive correlation between the number of c-Fos-positive cells and the amount of NREM sleep (Sherin et al., 1996; Lu et al., 2002). Consistently, electrophysiological recordings demonstrated that the firing rate of VLPO sleep-active neurons correlates with the depth of NREM sleep [indicated by electroencephalogram (EEG) delta power] (Szymusiak et al., 1998; Alam et al., 2014). Notably, VLPO sleep-active neurons displayed lower activity in the wake-sleep transition period than in the subsequent sleep episode (discharge rates further increased significantly from light to deep NREM sleep) (Szymusiak et al., 1998). These findings suggest that VLPO neurons might be involved in sleep maintenance, whereas other neurons may be responsible for sleep initiation. Using c-Fos activity approach, retrograde tracing, calcium imaging, and optogenetic and chemogenetic manipulations, we recently identified a population of POA-projecting glutamatergic neurons in the ventrolateral medulla (VLM) that controls the transitions from wakefulness to NREM sleep (Teng et al., 2022).
Here, we describe a detailed protocol for deep brain calcium imaging in the brainstem with simultaneous EEG/electromyography (EMG) recording to monitor neuronal activity during wake and sleep cycles (Figure 1). Compared to fiber photometry recording, this microendoscopic calcium imaging technique provides single-cell resolution to distinguish the function of subpopulations of VLM neurons in behaving mouse. Compared to in vivo electrophysiological recording, this imaging method allows the examination of neuronal activity in genetically defined cells. Similar in vivo calcium imaging techniques have been used to study sleep-related neuronal activity in other brain regions, including galanin-expressing GABAergic neurons in the dorsomedial hypothalamus (Chen et al., 2018) and neurotensinergic neurons in the midbrain (Zhong et al., 2019). A detailed imaging protocol in the cortical and subcortical areas has been published (Resendez et al., 2016). However, imaging the brainstem in freely moving animals typically involves significant movement artifacts. To overcome this issue, a previous study used anchoring tungsten wires glued to the gradient refractive index (GRIN) lens to stabilize the field of view (Gong et al., 2020). Here, we adopted this method to image VLM neurons in wake and sleep cycles. The whole experimental procedure takes approximately two months (Figure 1A). Our imaging protocol should be applicable to other deep brain regions that are involved in sleep regulation.
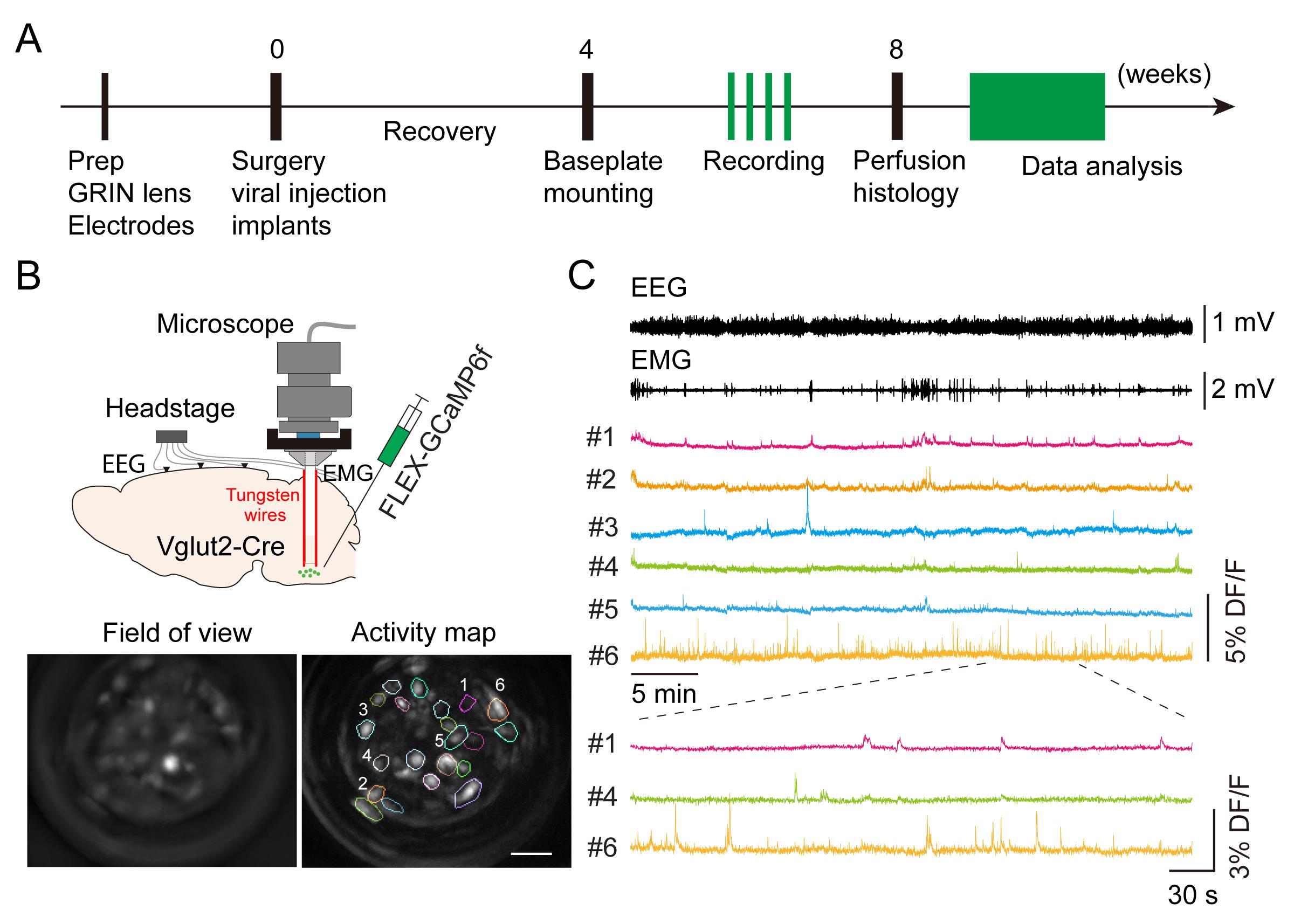
Figure 1. Imaging medulla glutamatergic neurons during sleep. A. Timeline of experimental design. B. Top: schematic of microendoscopic calcium imaging in the brainstem. Tungsten wires attached to the gradient refractive index (GRIN) lens were used to stabilize the field of view. Bottom: averaged GCaMP fluorescence image in the field of view and activity map of a representative imaging session in a Vglut2-Cre mouse. Scale bar, 50 μm. C. Example data showing electroencephalogram (EEG), electromyography (EMG), and representative calcium traces (DF/F) in an imaging session. Bottom: enlarged window showing calcium activity in three cells.
Materials and Reagents
Materials
GRIN lens and adapter, 0.5 mm diameter, 8.4 mm length (Inscopix, catalog number: 100-004155, adapter included)
Base plate (Inscopix, catalog number: 100-004096)
Base plate cover (Inscopix, catalog number: 100-002388)
5-position connectors (Mouser Electronics, catalog number: 437-8618300510001101)
Bone screws (J.I. Morris, catalog number: F000CE094)
Stainless wires, insulated, 0.003" diameter (A-M Systems, catalog number: 791100)
Silver wires, insulated, 0.005" diameter (A-M Systems, catalog number: 786000)
Flexible piano wires, 0.004" diameter (Precision Fiber Products, catalog number: SMWL-004-01)
Stainless steel custom-built headpost
Reagents
AAV1-syn-FLEX-GCaMP6f (Addgene, catalog number: 100833-AAV1)
Mineral oil (Fisher Scientific, catalog number: O121-1)
Contemporary Ortho-JetTM BCA powder (Lang Dental Manufacturing, catalog number: 1530BLK)
Ortho-JetTM liquid (Lang Dental Manufacturing, catalog number: 1304CLR)
Kwik-Cast silicone sealant (WPI, catalog number: KWIK-CAST)
3MTM Scotch-Weld epoxy adhesive DP100 clear (3M, model: DP-100)
Krazy glue (Krazy Glue, catalog number: KG94548R)
Ophthalmic ointment (Akorn, catalog number: 59399-0162-35)
Ketamine (Butler animal health holding, catalog number: 071069)
Xylazine (Butler animal health holding, catalog number: 061035)
Povidone iodine (MedSupply Partners, catalog number: D02-1202)
Equipment
Micropipette puller (Sutter Instrument, model: P-1000)
Surgical microscope (Leica, model: M60)
Stereotaxic instruments (David Kopf Instruments, model 900-U)
Nanoliter 2020 injector (WPI, NANOLITER2020)
DC temperature controller (FHC, 40-90-8D)
Micro drill (WPI, model: OmniDrill35)
Micromanipulator (Sutter Instrument, model: MP-285A)
Microendoscopic calcium imaging system (Inscopix, model: nVista3)
Electrophysiological recording system (Neuralynx, model: Digital Lynx 4S)
MDF sound attenuating cubicle (Med Associates, model: ENV-018MD)
Custom-built head-fixation platform (Figure 2, see components below):
1" XYZ translation stage (Thorlabs, catalog number: PT3)
Small dual-axis goniometer, 1/2" distance to point of rotation (Thorlabs, catalog number: GN2)
C-clamp (Siskiyou, catalog number: CC-2)
Post, 1/2" diameter, 8" length (Thorlabs, catalog number: TR8)
Parallel clamp for Ø1/2" posts, #8 counterbore and 3/16" hex (Thorlabs, catalog number: RA360)
Right-angle clamp for Ø1/2" posts, 3/16" hex (Thorlabs, catalog number: RA90-P5)
Aluminum breadboard, 6" × 12" × 1/2", 1/4"-20 taps (Thorlabs, catalog number: MB612F)
Mini-series aluminum breadboard, 3" × 4" × 3/8*, 8-32 and 1/4"-20 high-density taps (Thorlabs, catalog number: MSB34)
Custom-built metal adapter for headpost
M2.5 socket head cap screws for headpost
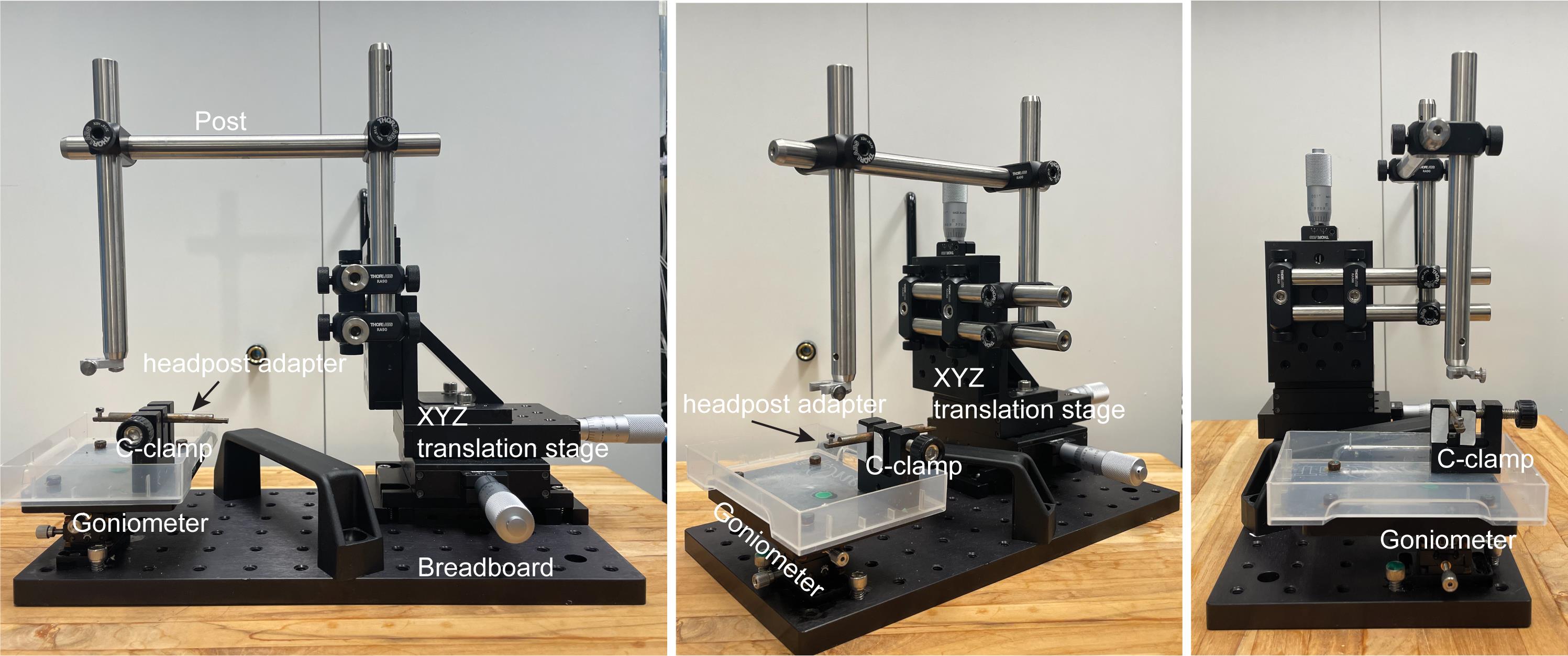
Figure 2. Head-fixation platform. Different views of the platform.
Software
Cheetah (Neuralynx, v5.7.4)
MATLAB (MathWorks, version 2021)
IDSP software (Inscopix, IDSP 1.80)
Procedure
文章信息
版权信息
© 2023 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Teng, S. and Peng, Y. (2023). Simultaneous Microendoscopic Calcium Imaging and EEG Recording of Mouse Brain during Sleep. Bio-protocol 13(9): e4664. DOI: 10.21769/BioProtoc.4664.
分类
神经科学 > 神经解剖学和神经环路 > 活细胞成像
神经科学 > 行为神经科学 > 睡眠与觉醒
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














